Table 4.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with a Phosphorus-Substituted Olefina
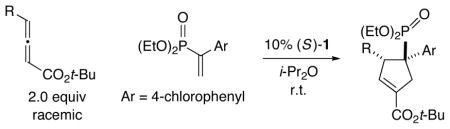 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | Me | 99 | 82 |
| 2 | CH2CH2Ph | 97 | 79 |
| 3 |
|
97 | 87 |
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Yield of purified product; for each cycloaddition, the ratio of regioisomers and diastereomers is ≥20:1 (determined by 1H NMR analysis of the unpurified reaction mixture).
