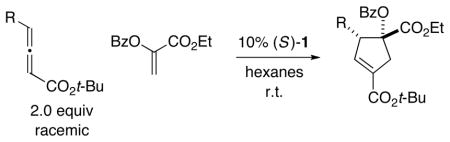
Table 5.
Catalytic Enantioselective [3+2] Cycloadditions of γ-Substituted Allenes with an Oxygen-Substituted Olefina
All data are the average of two experiments. For the determination of structure, including stereochemistry, see the Supporting Information.
Determined by 1H NMR analysis of the unpurified reaction mixture; for each cycloaddition, the ratio of regioisomers is ≥20:1.
Yield of purified product (dr ≥ 12: 1).