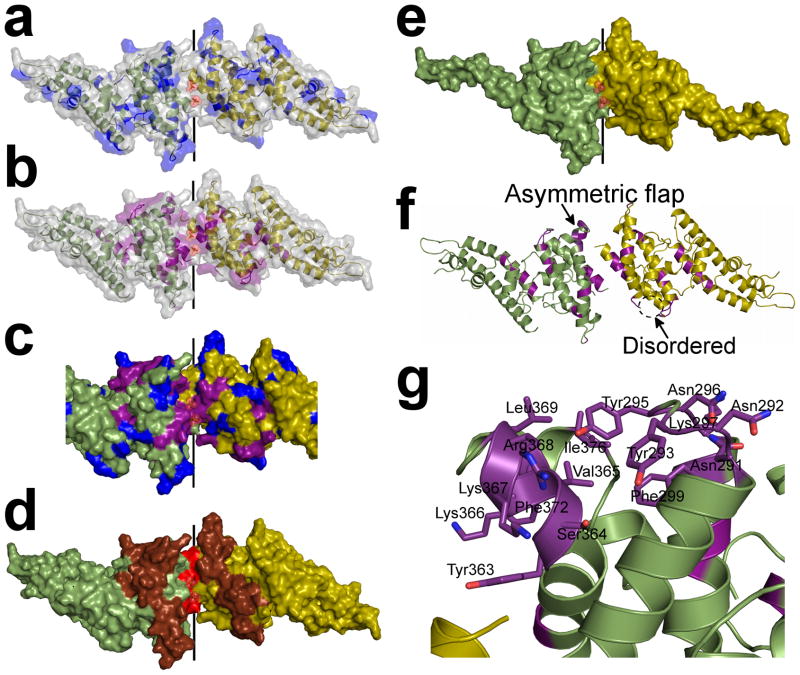
Figure 3.
The sulfotyrosine pocket, DARC binding groove and dimer interface are under selective pressure and are targeted by blocking-antibodies. Monomers are in green and yellow. (a) Polymorphic residues20,23 (blue) are excluded from the dimer interface but evenly distributed over the remaining RII-PvDBP surface. (b) Amino acid substitutions which abrogate RBC rosetting25,26 (purple) map to the DARC binding groove and dimer interface. (c) Overlay of polymorphic residues (blue) and critical receptor binding residues (purple) on the dimer. The DARC binding groove at the dimer interface is composed of essential residues and devoid of polymorphisms. (d) Epitopes recognized by blocking-antibodies22 (red – most significant, brown – significant) map to the functional regions of RII-PvDBP which include the dimer interface and DARC binding groove. (e) The minimal binding domain of RII-PvDBP (residues 256–426)27 contains the full dimer interface and DARC binding groove. (f) A global view of the dimer which shows the asymmetric flap is disordered in chain A. Essential residues are colored in purple. (g) A detailed view of the asymmetric flap shows this region contains several essential residues suggesting a second potential DARC binding site.