Abstract
Mitochondrial transcription factor A (Tfam) binds to and organizes mitochondrial DNA (mtDNA) genome into a mitochondrial nucleoid (mt-nucleoid) structure, which is necessary for mtDNA transcription and maintenance. Here, we demonstrate the mtDNA-organizing activity of mouse Tfam and its transcript isoform (Tfamiso), which has a smaller high-mobility group (HMG)-box1 domain, using a yeast model system that contains a deletion of the yeast homolog of mouse Tfam protein, Abf2p. When the mouse Tfam genes were introduced into the ABF2 locus of yeast genome, the corresponding mouse proteins, Tfam and Tfamiso, can functionally replace the yeast Abf2p and support mtDNA maintenance and mitochondrial biogenesis in yeast. Growth properties, mtDNA content and mitochondrial protein levels of genes encoded in the mtDNA were comparable in the strains expressing mouse proteins and the wild-type yeast strain, indicating that the proteins have robust mtDNA-maintaining and -expressing function in yeast mitochondria. These results imply that the mtDNA-organizing activities of the mouse mt-nucleoid proteins are structurally and evolutionary conserved, thus they can maintain the mtDNA of distantly related and distinctively different species, such as yeast.
Keywords: mitochondrial transcription factor A, Tfam, transcript isoform, mitochondrial nucleoid, mtDNA, ABF2
1. Introduction
Mitochondria are essential organelles that supply most of the energy for a cell through the generation of ATP by oxidative phosphorylation (Saraste, 1999). A unique feature of mitochondria is the presence of its own genome, known as the mitochondrial DNA (mtDNA) genome. The mtDNA encodes critical components of the ATP-generating pathway, as well as the rRNAs and tRNAs that are required for the mitochondrial translation system. Although all of the other genes needed for the biogenesis, maintenance and regulation of this organelle are encoded by the nuclear genome, mtDNA remains critical for normal mitochondrial function (DiMauro and Schon, 2008).
Although mitochondria do not contain histones, mtDNA is packaged into a protein-DNA complex known as a mitochondrial nucleoid (mt-nucleoid). Approximately 30 proteins from different species have been identified as potential components of the mt-nucleoid, including not only factors involved in DNA replication and transcription, but also structural proteins required for mtDNA maintenance (Chen and Butow, 2005). Major components of the mt-nucleoid complex are the non-histone, high mobility group (HMG) proteins such as the Abf2p protein from the yeast Saccharomyces cerevisiae and the mammalian Abf2p homologue TFAM (mitochondrial transcription factor A). These proteins show homology to the DNA-binding HMG proteins of nuclear chromatin (Landsman and Bustin, 1993).
In addition to packaging mtDNA into a nucleoid-like structure, TFAM is involved in mtDNA transcription, replication, protection and repair (Kang and Hamasaki, 2005). The human TFAM protein was initially cloned as a transcriptional activator of the mitochondrial genome and later found to be an essential factor for the maintenance of mtDNA (Alam et al., 2003; Kanki et al., 2004; Parisi and Clayton, 1991). TFAM enhances mtDNA transcription in the presence of mitochondrial RNA polymerase (POLRMT) and transcription factor B (TFBM) (Asin-Cayuela and Gustafsson, 2007; Scarpulla, 2008). Because replication of the mammalian mitochondrial genome is subsequently initiated following transcription, TFAM is also thought to be important for replication of mtDNA (Shadel and Clayton, 1997). These various functions require that this protein has both DNA-binding specificity that is related to its transcriptional function and non-specific DNA-binding properties. Mutational analysis of human TFAM revealed that the 25-residue carboxyl-terminal tail region is important for specific DNA recognition and essential for transcriptional activation (Dairaghi et al., 1995). The non-sequence-specific DNA-binding ability of human TFAM is mainly achieved by its first HMG-box, while the second HMG-box has low-DNA-binding capability (Wong et al., 2009). The DNA-binding ability of this protein is robust enough to cover entire regions of mtDNA due to its comparatively relaxed sequence specificity. In addition, TFAM stabilizes mtDNA through the formation of the mt-nucleoid complex and regulates (or titrates) the amount of mtDNA (Alam et al., 2003; Kanki et al., 2004).
The mtDNA-binding and organizing activities of the human TFAM protein have been experimentally demonstrated in vivo using a yeast model system, demonstrating its ability to complement a yeast abf2 deletion (Δabf2) mutant (Parisi et al., 1993). An HMG family protein, Abf2p, is abundant in yeast mitochondria at an estimated ratio of 1 molecule for every 15–30 bp of mtDNA, which is sufficient to bind the entire mitochondrial genome (Chen and Butow, 2005; Diffley and Stillman, 1992). Unlike mammalian TFAM proteins, Abf2p does not seem to be essential for either transcriptional initiation or replication of mtDNA (Diffley and Stillman, 1992), although deletion of the yeast ABF2 gene triggers rapid loss of mtDNA when the cells are grown on a fermentable carbon source (e.g., glucose) (Diffley and Stillman, 1991), suggesting that Abf2p is an architectural factor for organizing and maintaining the mitochondrial genome (Kanki et al., 2004). Glucose-containing medium allows cells to acquire energy through fermentation (glycolysis) and avoids the need for mitochondrial respiration. In turn, this process produces a respiratory-deficient petite mutant that has no mitochondrial genome. HMG-like proteins, such as human TFAM, the yeast nuclear HMG-like nonhistone proteins (NHP6A) and the bacterial histone-like DNA-binding Escherichia coli HU protein, which is not a member of the HMG family, are able to rescue the yeast Δabf2 strain by supporting replication of the yeast mitochondrial genome and providing the active mitochondrial respiration required for growth on non-fermentable (e.g., ethanol or glycerol) selective media (Kao et al., 1993; Megraw and Chae, 1993; Parisi et al., 1993). The functional complementation of yeast nucleoid proteins with the human TFAM and bacterial HU proteins is surprising given the low primary sequence homology of these proteins and the distinctively different features of various mitochondrial and bacterial genomes.
In this report, we demonstrate the mtDNA-organizing activity of mouse mitochondrial transcription factor A (Tfam) and its transcript isoform (Tfamiso) using a yeast model system that contains a deletion of the yeast homolog of mouse Tfam protein, Abf2p. Mouse Tfam protein is also composed of two tandem HMG-boxes that are connected by a linker sequence and a carboxyl-terminal tail that is rich in basic amino acid residues (Supplementary Fig. 1). Here we present data that the mouse mt-nucleoid protein Tfam supports yeast mtDNA for respiratory competence under a specific culture condition. In addition, we demonstrate that a mouse Tfam isoform (Tfamiso) that has a 17-amino acid deletion in the presumed HMG-box1 can also support maintenance of the yeast mitochondrial genome and rescue the loss-of-mtDNA phenotype in the Δabf2 mutant under a condition that requires active mitochondrial respiration.
2. Materials and methods
2.1. Strains and media
Synthetic minimal medium containing glucose (SD), synthetic minimal medium containing non-fermentable carbon sources such as ethanol and glycerol (SEG), complete medium containing glucose (YPD; 1% yeast extract, 2% peptone and 2% glucose), complete medium containing non-fermentable carbon sources (YPE: 1% yeast extract, 2% peptone and 3% ethanol; YPEG: 1% yeast extract, 2% peptone, 3% ethanol and 3% glycerol) and standard genetic manipulations were as described (Bonnefoy and Fox, 2002). Yeast S. cerevisiae MCC109 rho0 strain (MATα, ade2-101, ura3-52, kar1-1, ρ0) was obtained from Dr. Fox (Dunstan et al., 1997), and AH109 strain (MATa, trp1-901, leu2-3, ρ+) was obtained from Dr. Conklin (U of Minnesota).
2.2. Yeast transformation and DNA preparation
Transformation of yeast cells was performed by the lithium acetate (LiAc) method as described by Gietz and Schiestl (Gietz and Schiestl, 2007). Total cellular DNA, containing nuclear and mtDNA, from yeast was prepared by the lysis method according to Wach et al. (1994). Other recombinant DNA techniques were carried out as described by Sambrook et al. (Sambrook et al., 1989).
2.3. Construction of yeast strains with mouse Tfam or transcript isoform Tfamiso insertion
The mouse Tfam and Tfamiso genes were PCR-amplified from mouse IMAGE clones 1890394 and 21519111 (Invitrogen, Carlsbad, CA), respectively (Larsson et al., 1996; Marra et al., 1999). The Tfam transcript isoform (Tfamiso) contains a 66-nucleotide deletion after the mitochondrial targeting signal of the original Tfam gene (Supplementary Fig. 1). The Tfam and Tfamiso genes that were integrated into the yeast ABF2 locus by homologous recombination contained no Tfam mitochondrial targeting sequences. The mouse genes were placed after the sequence encoding the 26-amino acid yeast Abf2p mitochondrial leader peptide (Fig. 1). To recombine the Tfam or Tfamiso gene with a yeast selectable marker, the URA3 gene was also amplified by PCR, and recombinant PCR was performed to generate the Tfam-URA3 or Tfamiso-URA3 products. For chromosomal insertion, sequences corresponding to the yeast ABF2 leader pre-sequence followed by the coding portion of the mouse Tfam or Tfamiso protein were included. PCR primers were as follows: For Tfam, Tfamforward, 5′-CTTTCCACGAATCTTCTAAGCCCCTTTTCAATTTGGCTAGCACCTTGTTGTCCAGCATG GGTAGCTAT-3′ (ABF2 5′ homology through leader peptide underlined, 5′ end after leader peptide of Tfam italicized) and Tfamreverse, 5′-GAGTACCGCGGTCTAGTTGAGAGGTTAATGCTCAGAGATGTCTCC-3′ (URA3 homology for recombinant PCR underlined, 3′ end Tfam italicized). For Tfamiso, Tfamshortforward, 5′-CTTTCCACGAATCTTCTAAGCCCCTTTTCAATTTGGCTAGCACCTTGTTGTCCAAATTTA AAGCTAAACACCCA-3′ (5′ end of Tfamiso italicized) and the same reverse primer as for Tfam. For URA3, URA3forward, 5′-CAACTAGACCGCGGTACTCTCACAATGTTTTGATTCGGTAATCTCCGAGC-3′ (homology for recombinant PCR underlined) and URA3reverse, 5′-GAATAAAGGCATAAAAAACATTGTGAGAGTACCGCGGTCTAGTTGAGAGGAGAAATC ATTACGACCGAGATT-3′ (ABF2 3′ end homology underlined). ABF2 assay PCR (Fig. 1 and Fig. 3B) was performed to ensure deletion of the ABF2 gene using the following primers: ABF2 internal, 5′-GATAAATGGCAATCCTTGGATC-3′ and ABF2assay3′, 5′-GGTGAGGACGAGTTATGGTG-3′. The Tfam and Tfamiso insertions were also confirmed by PCR using the following primers (Fig. 1 and Fig. 3B): for Tfam, Tfamforward and ABF2assay3′ primers; for Tfamiso, Tfamshortforward and ABF2assay3′ primers. As a control, MGM1 assay PCR was performed using primers 5′-GCCTTGCTCGTCAACATTCC-3′ and 5′-GCCTTGAAATCTTCGGATCTTCC-3′ (Fig. 3B). The abf2 deletion mutants with and without mitochondrial genomes (Δabf2 ρ+ and Δabf2 ρ0) were generated by replacing ABF2 with URA3, followed by PCR amplification using the primers Abf2-5′homology-5′URA3 (5′-CTTTCCACGAATCTTCTAAGCCCCTTTTCAATTTGGCTAGCACCTTGTTGCTGAGAGT GCACCATACCAC-3′) and 3′URA3-Abf2-3′homology (5′-GAATAAAGGCATAAAAAACATTGTGAGAGTACCGCGGTCTAGTTGAGAGGGGGTAAT AACTGATATAATTAAATTGAAG-3′).
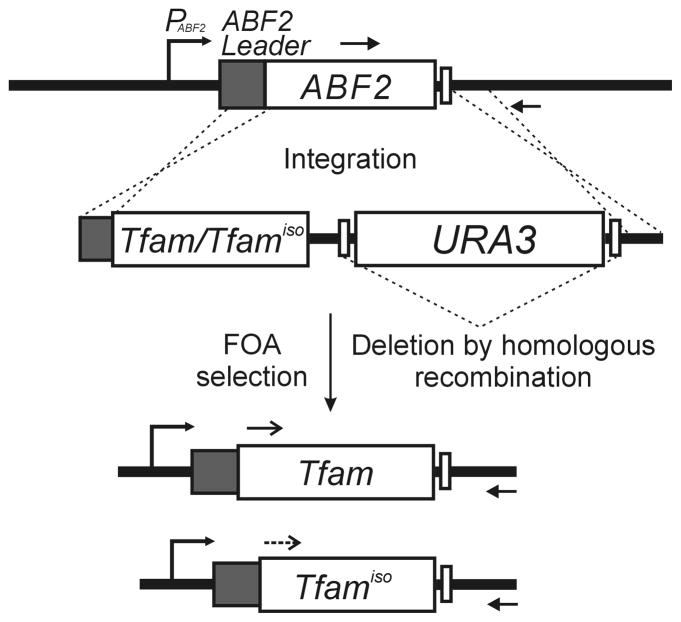
Figure 1.
Integration of mouse Tfam and the transcript isoform Tfamiso into the yeast ABF2 locus. Tfam or Tfamiso without the mouse mitochondrial targeting sequence was inserted into yeast ABF2 by homologous recombination. The mouse genes were placed after the sequence encoding the 26-amino acid yeast mitochondrial leader peptide (ABF2 leader). Arrows indicate the PCR assay primers for detecting the correct integration of the Tfam and Tfamiso genes and deletion of the ABF2 gene. After integration was confirmed, the URA3 marker gene was removed by FOA-mediated homologous recombination.
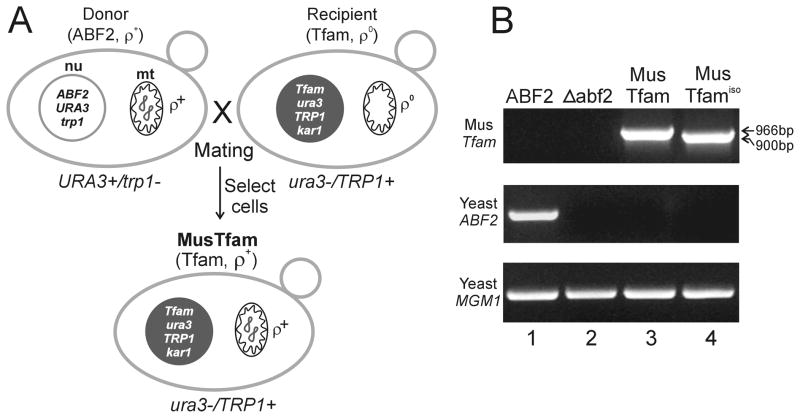
Figure 3.
Construction of strains that maintain the yeast mitochondrial genome using mouse mtDNA-packaging proteins. (A) Construction of a yeast strain carrying the mouse Tfam gene and the entire yeast mtDNA. The kar1-1 mutation and other selection markers can be used to select the yeast strain (Tfam, ρ+) that maintains active mitochondrial respiratory function after a genetic cross (mating) between the wild-type mtDNA-donor (ABF2, ρ+) and Tfam ρ0-recipient strains. (B) PCR analysis. After generating MusTfam and MusTfamiso strains through the mating and selection process, insertion of the mouse Tfam and Tfamiso genes and deletion of the yeast ABF2 gene were confirmed using PCR (see Figure 1 for primer pairs). As a control, assay PCR for the yeast MGM1 gene was performed. Lane 1, ABF2 (MCC109 ρ+) wild-type; lane 2, Δabf2 (MCC109 abf2 deletion) mutant; lane 3, MusTfam (MCC109 ρ+ Tfam) strain; lane 4, MusTfamiso (MCC109 ρ+ Tfamiso) strain.
2.4. Construction of Tfam-GFP fusion
To generate a GFP fusion with Tfam, GFP was amplified using the primers 5′-ACGAAGTGATCTCATCCGTCGAAGTGTGAAACGATCCGGAGACATCTCTGAGCATATG GTGAGCAAGGGCGAGGA-3′ (mouse Tfam homology underlined, 5′ end of the GFP italicized) and 5′-GAGTACCGCGGTCTAGTTGAGAGGTTACTTGTACAGCTCGTCCA-3′ (homology for recombinant PCR underlined, 3′end of the GFP italicized) and recombined with URA3 to produce a GFP-URA3 fusion gene. The recombinant product was transformed into the mouse Tfam strain to induce homologous recombination into the 3′end of the Tfam gene. The ABF2Leader-musTfam-GFP fusion was then amplified using primers 5′-ATCGGATCCATGAACAGTTACAGCCTATTAAC-3′ and 5′-AGTGTCGACTTACTTGTACAGCTCGTCCA-3′ and cloned into a BamHI- and SalI-digested pG-1 vector (Schena et al., 1991).
2.5. Real-time PCR to determine yeast mtDNA content
ABI Prism 7000 sequence detection system (Applied Biosystems) was used for analysis. Total yeast cellular DNA of 50 ng was used for assaying mtDNA and nuclear DNA markers, respectively, in a 25 μl reaction containing SYBR Premix Ex Taq, ROX reference dye (Takara, Shiga, Japan) and 100 nM each of primers. The COX2 and MGM1 genes were used for yeast mtDNA and nuclear DNA markers, respectively. The primer sequences were as follows: for COX2, 5′-TCAGGATTCAGCAACACCAAATCAAGA-3′ and 5′-TGGCATATTTGCATGACCTGTCCCA-3′; for MGM1, 5′-GCCTTGCTCGTCAACATTCC-3′ and 5′-GCCTTGAAATCTTCGGATCTTCC-3′. Reactions were repeated at least twice per experiment and experiments were repeated three times to verify results. The amplification program used was as follows: initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 30 sec, 55°C for 1 min and 72°C for 70 sec, with a single fluorescence measurement per cycle. Quantification was performed by interpolation of a standard curve of threshold cycle (Ct) values generated from serially diluted DNAs derived from pooled samples. The Ct values of the MGM1 and the mitochondrial COX2 genes were determined in each real-time PCR reaction. The mtDNA content was then normalized with the content of the MGM1 gene to calculate the relative mtDNA content.
2.6. Western blot analysis
To prepare total cellular extracts, yeast cells were cultured at 30°C in 5 ml of YPE medium overnight. These cultures were inoculated into 50 ml of YPD or YPE medium and the incubation was continued at 30°C for 10 hr by shaking the culture at 200 rpm. The cells were then harvested by centrifugation at 3,000 × g for 20 min, washed with 1 ml of ice-cold water, and resuspended in 300 μl of cracking buffer (8 M urea, 5% SDS, 40 mM Tris-HCl, pH 6.8, 0.1 mM EDTA, 3.3 μl of 2-mercaptoethanol, with 70 μl of protease inhibitor cocktail (Sigma) and 2 mM phenylmethylsulfonyl fluoride). About 0.5 ml of 425–600 μm acid-washed glass beads (Sigma) were added to each tube, and the cells were lysed by vortexing four times for 45 sec with 30-sec intervals on ice. Glass beads and unbroken cells were removed by centrifugation at 3,000 × g for 10 min and remaining supernatants were analyzed by SDS/PAGE and Western blotting. The Cox1p, Cox2p (Mitosciences, Eugene, OR) and Pgk1p (Invitrogen) primary mouse monoclonal antibodies for yeast were used at a 1:1,000 dilution and the secondary anti-mouse IgG conjugated with horseradish peroxidase (HRP) was used at a 1:2,000 dilution. Immuno-reactivity was visualized by SuperSignal West Pico enhanced chemiluminescence substrate (Pierce, Rockford, IL) and detected with LAS-3000PLUS system (Fuji Photo Film Company, Kanagawa, Japan).
3. Results
3.1. Insertion of the mouse Tfam genes at the ABF2 locus
To introduce the mouse Tfam genes (Tfam and Tfamiso) into the yeast ABF2 locus, PCR-mediated recombination of the Tfam genes, along with deletion of the ABF2 gene, was performed using yeast ρ0 cells (MCC109 ρ0) that have no mitochondrial genomes in their mitochondria. The mouse Tfam coding sequence and the transcript isoform Tfamiso were isolated from mouse liver and kidney cDNA, respectively (Fig. 1) (Marra et al., 1999). The Tfamiso protein has a 17-amino acid deletion in the HMG-box1 region (Supplementary Fig. S1). The Tfam and Tfamiso genes without the mouse mitochondrial leader sequences were amplified and recombined with the URA3 selection marker by recombinant PCR to generate Tfam-URA3 and Tfamiso-URA3, respectively (Fig. 1). Approximately 50- to 52-nucleotide regions of homology from the mitochondrial leader sequence and 3′-UTR region of the yeast ABF2 gene were added to the flanking regions of these products, which enabled the recombinant PCR products to be integrated into the ABF2 locus by homologous recombination (Fig. 1).
The Tfam-URA3 and Tfamiso-URA3 constructs were transformed into haploid yeast MCC109ρ0 cells, and Ura+ transformants were selected on synthetic glucose minimal medium without uracil (SD-Ura). Chromosomal integration of the constructs was analyzed by PCR. DNA sequencing of the PCR products was also performed to confirm the correct sequence insertion (data not shown). The Ura+ transformants were then cultured in complete glucose medium (YPD) and spread on FOA-containing synthetic complete medium to remove the URA3 marker gene (Fig. 1) (Boeke et al., 1987). The addition of the 36-nucleotide sequence duplications (5′-CCT CTC AAC TAG ACC GCG GTA CTC TCA CAA TGT TTT-3′) flanked by the URA3 gene facilitated the elimination of the marker gene by homologous recombination between these sequence duplications (Fig. 1).
3.2. Tfam targeting into mitochondria
To confirm the mitochondrial targeting of mouse Tfam into yeast mitochondria, a yeast vector expressing a Tfam-GFP fusion protein was constructed. The yeast mitochondrial leader sequence from the ABF2 gene was fused in-frame with the mouse Tfam-GFP coding sequences (see Materials and Methods). When this expression vector was transformed into wild-type yeast cells, the expression of the Tfam-GFP protein resulted in a punctuate fluorescence pattern located near the periphery of the cells (Fig. 2), which is similar to the typical morphological fluorescence pattern seen when expressing Abf2p-GFP (Chen and Butow, 2005; Zelenaya-Troitskaya et al., 1998). Because the Tfam-GFP protein expressed from the vector contains the mitochondrial leader sequence of the yeast Abf2p and the punctuate fluorescence pattern closely matched the pattern obtained by staining with a mitotracker (Supplementary Fig. S2), this fluorescence pattern indicates that the Tfam protein can be efficiently and correctly targeted to yeast mitochondria.
Figure 2.
Localization of mouse Tfam to yeast mitochondria. The Tfam-GFP fusion gene was cloned into a yeast expression vector and used to transform wild-type yeast cells. Tfam-GFP displayed a spotty fluorescence pattern located near the periphery of these cells, which is identical to the pattern seen in yeast cells with the yeast homolog Abf2p-GFP fusion protein.
3.3. Construction of yeast strains that maintain active mitochondrial respiration function in the absence of yeast Abf2p
To test whether the mouse Tfam and Tfamiso proteins are able to functionally replace yeast Abf2p, intact yeast mitochondrial genomes (ρ+) were introduced into these Tfam and Tfamiso ρ0 yeast strains. Because the strain MCC109 carries the karyogamy-defective mutation (kar1-1), which allows efficient mitochondrial fusion but reduces nuclear fusion (Conde and Fink, 1976), the Tfam-inserted strain (MCC109 ρ0 Δabf2::Tfam or MCC109 ρ0 Δabf2::Tfamiso, MATα) was mated with a wild-type yeast strain to transfer the ρ+ yeast mitochondrial genome (Fig. 3A). The wild-type AH109 strain (MATa, ρ+), which cannot grow on tryptophan-deficient media, was used as the yeast mitochondrial genome donor. The recipient cells (Tfam-inserted strains), which have no mitochondrial genomes due to the nature of ρ0 cells, cannot grow on media with non-fermentable carbon sources, which requires mitochondrial respiration for growth. The mating mixtures were then spread on synthetic tryptophan-deficient selective medium containing non-fermentable carbon sources (SEG-trp). In this selective medium, both haploid (MCC109Tfam ρ+ or MCC109Tfamiso ρ+) cells, which are generated due to the karyogamy-defective mutation (kar1-1), and diploid (MCC109Tfam ρ0×AH109 ρ+ or MCC109Tfamiso ρ0×AH109 ρ+) cells, which are generated at low efficiency, can grow.
To identify ploidy of the cells that were grown on this selective medium, each of the candidate yeast colonies were re-streaked on synthetic uracil-deficient medium (SEG-ura). Since the mtDNA donor strain (AH109 ρ+) can grow on media without uracil, cells that can grow on both SEG-ura and SEG-trp media after mating are typically diploid, while haploid cells containing mitochondrial genomes (MCC109Tfam ρ+ or MCC109Tfamiso ρ+) can only grow on tryptophan-deficient selective medium (SEG-trp) but not on uracil-deficient medium (SEG-ura). This method can, therefore, be used to selectively screen for haploid yeast strains carrying the intact yeast mitochondrial genome with the mouse Tfam or Tfamiso insertion (designated as the MusTfam or MusTfamiso strain). The Tfam and Tfamiso insertions at the ABF2 locus in these haploid strains were confirmed by PCR analysis (Fig. 3B). Tfam PCR analysis showed 966- and 900-bp products in MusTfam and MusTfamiso strains, respectively (Fig. 3B, top panel, lanes 3 and 4), whereas no products were obtained in the Abf2 assay PCR (Fig. 3B, middle panel, lanes 3 and 4). These results indicated that each of the Tfam genes was correctly integrated into the ABF2 locus and replaced the yeast ABF2 gene and that the genetic method using the kar1-1 mutation to generate haploid yeast cells after mating was reliable in this experimental system.
3.4. Growth properties of MusTfam and MusTfamiso strains
We next investigated the complementation of yeast Abf2p function with mouse Tfam and Tfamiso proteins by testing the growth properties of the yeast strains in media containing non-selective fermentable carbon source (e.g. glucose, YPD) or selective non-fermentable carbon source (e.g. ethanol, YPE) (Fig. 4). The growth phenotypes of the MusTfam and MusTfamiso strains on YPD medium were essentially identical with that of the ABF2 (wild-type) cells (Fig. 4A), which was also confirmed by measuring the cell growth at time intervals (Fig. 4C). During incubation, all strains, including the abf2 deletion mutant with mtDNA (Δabf2 ρ+), grew equally well in the glucose medium (YPD). However, the growth of the abf2 deletion mutant with no mtDNA (Δabf2 ρ0) was apparently delayed compared to that of the Δabf2 ρ+, which possibly resulted from the complete loss of mitochondrial genomes (Fig. 4A).
Figure 4.
Yeast mtDNA-supporting activity of mouse Tfam and its transcript isoform (Tfamiso). Mouse mitochondrial proteins can functionally replace the mtDNA-organizing activity of the yeast homolog Abf2p. (A and B) Growth phenotypes. The growth phenotypes of the wild-type (ABF2), MusTfam and MusTfamiso strains were very similar when grown in glucose medium (YPD). The MusTfam and MusTfamiso strains were able to grow on ethanol medium (YPE), on which the cells need active mitochondrial function for growth. Although the growth rates of the wild-type, MusTfam and MusTfamiso strains on the ethanol medium seemed to be different, according to their colony sizes, all of these strains grow healthy and without any difficulties. The abf2 deletion mutant that lost its mitochondrial genome (Δabf2 ρ0) cannot grow on ethanol medium due to the absence of active mitochondrial function. In contrast, the abf2 deletion mutant that maintained its mitochondrial genome (Δabf2 ρ+) was able to grow on ethanol medium, but the growth of the strain was very slow and defective. (C) Cell growth curves of the indicated yeast strains in glucose medium (YPD). 50 ml of glucose medium inoculated with each strain was cultivated in a shaking incubator at 30°C. Samples were removed from each culture at intervals and the absorbance at 600 nm (OD600) was measured. (D) Cell growth curves of the indicated yeast strains in ethanol medium (YPE). Each strain was cultured in 50 ml ethanol medium and the absorbance at 600 nm (OD600) was measured at intervals as in panel C.
In a medium containing a non-fermentable carbon source (YPE), cells must maintain active mitochondrial function for growth and thus are required to retain mitochondrial genomes in their mitochondria. The MusTfam and MusTfamiso strains were able to maintain mitochondrial respiration function, as demonstrated by their ability to grow on the YPE medium (Fig. 4B and 4D). In contrast, the Δabf2 ρ0 strain could not grow, due to loss of mtDNA (Fig. 4B) (Diffley and Stillman, 1991; Sia et al., 2009). The Δabf2 ρ+ could also grow on the ethanol medium, but the sizes of the colonies were much smaller than those of the colonies from the MusTfam and MusTfamiso yeast strains (Fig. 4B). Ethanol growth curves revealed the rank order of growth among these strains (Fig. 4D). The MusTfam and MusTfamiso strains were able to propagate rapidly, whereas the Δabf2 ρ+ strain grew very slowly for generations, presumably because of defective mitochondrial function due to loss of Abf2p activity. These observations indicate that the mouse Tfam and Tfamiso proteins provide yeast mtDNA-supporting activity in the abf2 deletion background and thus improve the growth properties in the ethanol medium. However, the growth of the MusTfam and MusTfamiso strains in the ethanol medium were relatively slower than that of the wild-type (ABF2) yeast cells (Fig. 4B and 4D). The latter observation is presumably due to the relaxed interactions between Tfam proteins and the yeast mitochondrial genome. From these results, we concluded that the mouse Tfam and Tfamiso proteins can maintain yeast mtDNA-organizing activity for respiratory competence in yeast mitochondria and thus complement yeast Abf2p function.
3.5. Relative mtDNA content in MusTfam and MusTfamiso strains
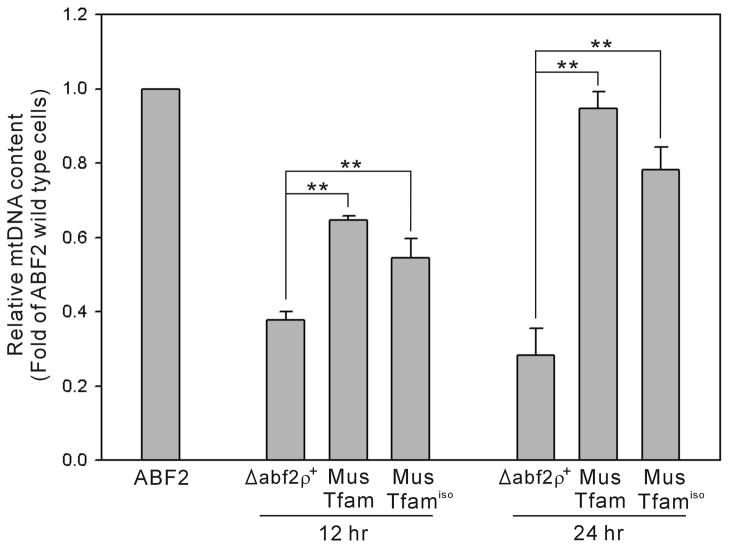
Even though MusTfam and MusTfamiso strains can grow on the respiration-requiring ethanol medium, their log-phase growth was relatively slower than the wild-type (ABF2) strain (see Fig. 4B). To investigate the differences in growth properties, we analyzed mtDNA levels in these strains. The yeast strains were cultured for 12 and 24 hr in medium containing ethanol (YPE) to maintain mitochondrial respiration function, and then total genomic DNA was isolated. Yeast mtDNA content relative to nuclear DNA was determined by real-time PCR. The MGM1 gene (Meeusen et al., 2006), a single-copy nuclear gene, was employed as the target gene for the determination of nuclear DNA abundance, whereas the mitochondrial COX2 gene (Williamson, 2002) was used in the determination of mtDNA content. The relative mtDNA contents of MusTfam and MusTfamiso strains increased during the culture (Fig. 5). At the 24 hr time point, the mtDNA level of MusTfam strain reached that of the wild-type (ABF2) cells. The mtDNA level of the MusTfamiso strain was slightly lower (~15%) than that of the MusTfam strain at both 12 and 24 hrs. These results are in good agreement with the growth properties, which demonstrate the slower growth of the recombinant strains compared to the wild-type yeast cells (Figs. 4B and 4D). The Δabf2 ρ+ strain could also maintain mtDNA when grown in this non-fermentable medium containing ethanol, but the mtDNA levels at the 12 and 24 hr time points were significantly lower than those of strains containing Abf2p (wild-type ABF2) or Tfam (MusTfam and MusTfamiso) proteins, presumably due to the instability of mtDNA in this strain (Sia et al., 2009).
Figure 5.
MtDNA content of the yeast strains grown in respiration-requiring media. Total genomic DNA from wild type (ABF2), MusTfam, MusTfamiso and Δabf2 ρ+ strains was prepared after culturing the cells in ethanol medium (YPE) for 12 and 24 hr at 30°C in a shaking incubator. Real-time PCR was used to determine yeast mtDNA content relative to nuclear DNA. At both 12 and 24 hr time points, the relative mtDNA contents in MusTfam and MusTfamiso strains were significantly increased compared to Δabf2 ρ+ strain (**P<0.01).
3.6. Expression of yeast mitochondrial proteins encoded by mtDNA
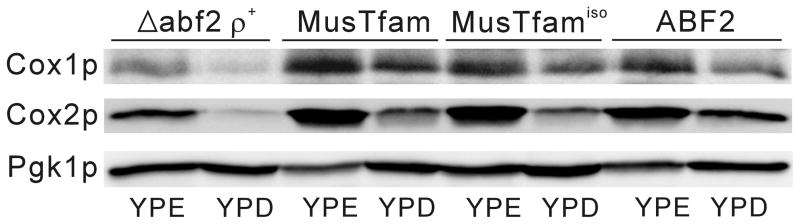
Because the mouse nucleoid proteins Tfam and Tfamiso supported maintenance of mtDNA in yeast mitochondria and allowed mitochondrial respiratory function in cells (see Figs. 4 and 5), we further tested whether the Tfam proteins could also support the expression of yeast mitochondrial proteins encoded by the mitochondrial genome. Yeast mitochondrial genomes contain eight protein-coding genes, three ribosomal RNA genes and 24 transfer RNA genes (Williamson, 2002). Among these genes, we assayed the expression of the COX1 and COX2 genes by western blot because these two genes are distantly located on the yeast mitochondrial genome (~47 kb apart) and are involved in one of the complexes of oxidative phosphorylation (Jacobs, 2001; Williamson, 2002). The yeast cells were cultured in media containing either ethanol (YPE) or glucose (YPD) for 12 hr and total yeast proteins were prepared by disrupting cells with the glass beads method. As shown in Fig. 6, the expression patterns of Cox1p and Cox2p proteins in the MusTfam and MusTfamiso strains were comparable to those of the wild-type (ABF2) cells when cultured in YPE and YPD. In contrast, the expression levels of the mitochondrial proteins were clearly decreased in the Δabf2 ρ+ strain compared with those in the MusTfam and MusTfamiso strains. Interestingly the expression levels of Cox1p and Cox2p in YPD cultured cells were reduced compared with those in YPE medium, which was observed in all strains, including the wild-type.
Figure 6.
Analysis of the mitochondrial proteins expressed from yeast mitochondrial genomes. Δabf2 ρ+, MusTfam, MusTfamiso and wild-type (ABF2) yeast cells were cultured in ethanol (YPE) and glucose (YPD) media, respectively, and total protein isolated from the cells was fractionated in a SDS/PAGE gel. Western blot analysis was performed using Cox1p, Cox2p and Pgk1p monoclonal antibodies for yeast. The expression levels of the mitochondrial proteins (Cox1p and Cox2p) in MusTfam and MusTfamiso strains in each medium are similar to those of the wild-type cells. Pgk1p was used as a loading control.
4. Discussion
Although the Δabf2 ρ+ mutant cells rapidly lost their mitochondrial genomes when the cells were cultured in a medium containing a fermentable carbon source, mtDNA could be maintained in these cells when grown in a medium containing a non-fermentable carbon source such as ethanol or glycerol (Diffley and Stillman, 1991; Miyakawa et al., 2010). However, under culture conditions with a non-fermentable carbon source, which requires mitochondrial gene expression, the Δabf2 ρ+ mutants exhibited a growth defect (Diffley and Stillman, 1991; Sia et al., 2009). Recently it was reported that mtDNA in the Δabf2 mutant strains displayed higher rates of mutation, such as mitochondrial frameshifts and direct-repeat mediated deletions, which eventually resulted in the destabilization of mtDNA maintenance (Sia et al., 2009). Thus, the yeast ABF2 gene seems to be important for both the maintenance and the expression of the mitochondrial genome.
MtDNA instability in Δabf2 cells can be suppressed by HMG proteins such as the human mt-nucleoid protein, TFAM, and the non-HMG E. coli DNA-packaging protein, HU, when they are targeted to mitochondria (Megraw and Chae, 1993; Parisi et al., 1993). With regard to these studies, we employed the Δabf2 mutants to explore the mtDNA-supporting activity of the mouse Tfam protein and its transcript isoform in stabilizing the yeast mitochondrial genome and rescuing the loss-of-mtDNA phenotype of the Δabf2 yeast strain. In this study, we tested the functional complementation of yeast Abf2p activity with a transcript isoform (Tfamiso) that has a small deletion in the HMG-box1 region of mouse Tfam protein (Supplementary Fig. 1). A report on a smaller isoform of human TFAM produced by alternative splicing demonstrated that, in addition to the major TFAM mRNA, 30% of the mRNA in the cells contained a 96-bp deletion in the region of the second HMG-box and that this smaller mRNA was found in most human tissues (Tominaga et al., 1993). The mouse Tfam gene has also been reported to be alternatively spliced and broadly expressed in tissues (Larsson et al., 1996). Thus, different isoforms of Tfam proteins, which are either expressed in a broad range of tissues or in a tissue-specific manner, may play an important role in the concerted regulation of mitochondrial genomes during mitochondrial biogenesis (Tominaga et al., 1993). Therefore, we investigated whether the alternatively spliced transcript isoform of Tfam has mtDNA-supporting activity in a yeast model system. The alternatively spliced form (Tfamiso) used in this experiment was isolated from a kidney cDNA library (Marra et al., 1999) and contained a 66-nucleotide deletion after the mitochondrial targeting signal. This deletion generates an HMG-box1 with 17 fewer amino acids than that of the full-length Tfam protein (Supplementary Fig. 1).
To test yeast mtDNA-organizing activity of mouse mt-nucleoid proteins, the full-length mouse Tfam gene or its transcript isoform (Tfamiso) was inserted into the yeast ABF2 gene locus. Growth properties demonstrated that the yeast strains with the full or alternatively spliced Tfam gene (MusTfam or MusTfamiso) could grow well on plates with a non-fermentable carbon source (ethanol), indicating that these cells can replicate and transcribe yeast mitochondrial genomes to support mitochondrial biogenesis and thus enhance growth in ethanol media (Fig. 4B). Although the growth rate of the MusTfam strain, based on the colony sizes, was slightly slower compared to that of the wild-type strain in an ethanol medium, the MusTfam cells appeared very healthy and grew vigorously in this non-fermentable medium (Fig. 4B). The MusTfamiso colonies, however, showed delayed growth compared to the wild-type and the MusTfam cells (Fig. 4B). The difference in growth properties seen in the MusTfamiso strain is probably because the Tfamiso protein contains a deletion in the HMG-box1, a domain that contributes to non-specific DNA binding capability, likely resulting in loose interaction of Tfamiso proteins in maintaining mtDNA and/or structuring mitochondrial nucleoids in yeast mitochondria. According to our experiments with regard to the mtDNA-organizing activity using a yeast model, this transcript isoform could promote respiratory competence of Δabf2 cells by supporting yeast mtDNA maintenance and expression (see Figures 5 and 6). The mtDNA-supporting function of this isoform, however, was relatively weaker than the full length Tfam protein, which might be necessary for maintaining concerted regulation of mtDNA transcription and replication in mouse cells (Bruno et al., 2007).
We next investigated mtDNA levels in MusTfam and MusTfamiso strains to determine whether yeast mtDNA was completely maintained when grown in the respiration-requiring ethanol medium (see Fig. 5). Fig. 4B and 4D indicate a slower growth compared to the wild-type (ABF2) cells and an apparently slower growth of the shorter isoform (Tfamiso) compared with the full-length Tfam, likely due to the relative mtDNA content of the MusTfam and MusTfamiso strains being lower than the wild-type yeast cells (Fig. 5). MtDNA levels in the MusTfam strain were ~65% at 12 hr of culture compared to the wild-type cells, and at 24 hr the level of mtDNA reached a nearly equivalent level (~95%) compared to the wild-type cells, suggesting that the expression of the mouse Tfam gene in yeast stabilizes the yeast mtDNA gradually and so in effect functionally replaces the activity of its yeast homolog. MtDNA levels in the MusTfamiso strain were ~55% and ~80% compared to the wild-type strain in 12 and 24 hr cultures, respectively, which is consistent with its slower growth properties in the ethanol medium. In contrast, the mtDNA content of the Δabf2 ρ+ strain was far less than that of the MusTfam and MusTfamiso strains, which is in agreement with the defective growth of the Δabf2 ρ+ cells grown in YPE medium (Fig. 4) (Diffley and Stillman, 1991; Sia et al., 2009). These results indicate that the mouse mt-nucleoid proteins can maintain yeast mtDNA in a stable conformation that can be shifted to the respiratory condition properly in the abf2 deletion background.
Replication and maintenance of the complete yeast ρ+ mitochondrial genome requires mitochondrial protein synthesis through the process of mitochondrial transcription and translation. Although the overall genetic organization and even the genetic code used can differ radically in the mitochondrial genomes of eukaryotes that are more distantly related to mammals, the molecular components of the mitochondrial transcriptional machinery are proving to be highly analogous in most eukaryotic species (Bonawitz et al., 2006; Shadel, 1999). Unlike mammalian mitochondrial genomes, which have only two major transcriptional promoters (Clayton, 2000), at least 16 distinct transcripts are generated from the yeast mtDNA (Biswas, 1998). Each of these transcripts initiates at its own independent promoter, which consists of a simple nonanucleotide consensus sequence. Despite these organizational differences, yeast has homologs of the mammalian mitochondrial transcription machinery that consists of POLRMT and TFBM (Rpo41p and Mtf1p in yeast), as well as TFAM (Abf2p) (Asin-Cayuela and Gustafsson, 2007; Scarpulla, 2008). Although Abf2p does not appear to play a direct role in initiating transcription in yeast, Rpo41p and Mtf1p are thought to both serve the same general role as their mammalian homologs. Therefore, we investigated the expression of mitochondrial proteins encoded from mtDNA in the MusTfam and MusTfamiso strains to test whether the gene expression activity of mouse Tfam is preserved in yeast lacking Abf2p, which would indicate that mouse Tfam proteins can function in the process of yeast mitochondrial gene expression instead of Abf2p. When cultured in YPE medium that requires active mitochondrial respiratory function for growth, the expression levels of Cox1p and Cox2p, encoded by yeast mtDNA, in the MusTfam and MusTfamiso strains were essentially indistinguishable from those in the wild-type (ABF2) strain (Fig. 6). This finding suggests that the interactions between Tfam proteins and mtDNA expression machinery are conserved, thus Tfam proteins can support gene expression from yeast mitochondrial genomes in the absence of Abf2p. Interestingly, when grown in YPD glucose medium, the expression levels of the mitochondrial proteins were reduced in all strains, including the wild-type (Fig. 6). The cause of this phenomenon could be that mitochondrial gene expression is controlled by the metabolic state, which depends on the environmental and nutritional conditions (Scarpulla, 2008). In glucose media, cells can acquire energy through glycolysis (fermentation) and thus avoid the process of mitochondrial respiration by altering the gene expression from the mitochondrial genomes.
The approach we described for mtDNA-supporting activity of mouse mt-nucleoid proteins in yeast offers an attractive experimental system for studying many aspects of mammalian mitochondrial genetics. Although the critical importance of mitochondrial genomes to human health is now well understood, no practical means have yet been found to directly modify mitochondrial sequences in mammalian cells for studying mitochondrial molecular genetics (Yoon and Koob, 2003). Yeast has in many ways proven to be an ideal model system in which to study mitochondrial molecular genetics (Shadel, 1999). Because yeast S. cerevisiae is one of the few species for which a mitochondrial transformation procedure has been developed and has highly active homologous recombination activity in mitochondria (Fox et al., 1988; Johnston et al., 1988; Pinkham et al., 1994), yeast mtDNA can be easily manipulated to insert exogenous DNA such as mouse or human mitochondrial transcriptional heavy and light strand promoters (HSP and LSP). Despite the unique and critical function that the HSP and LSP promoters serve in human physiology (Clayton, 2000), we know amazingly little about the role that either the mtDNA promoter sequences or proteins that interact with these sequences play in initiating transcription at these sites in vivo. Although many detailed understanding that we currently have concerning mitochondrial transcription initiation in mice and humans has come from in vitro transcription studies (Shadel and Clayton, 1993, 1997; Shutt et al., 2010; Shutt et al., 2011), in vivo study is essential in order to obtain a more detailed and biologically accurate understanding of mammalian mitochondrial transcription initiation. Our experimental system described here would allow us to extend these in vitro transcription studies to in vivo analysis by reconstituting the mammalian mitochondrial transcription in living cells. The component proteins that are known to involve in the transcription from the mitochondrial promoters in vitro are TFAM, POLRMT and TFBM (TFB1M or TFB2M). Since inclusion of either of TFBM together with POLRMT and TFAM in an in vitro transcription assay was shown to be necessary to initiate transcription from the mammalian HSP and LSP promoters (Falkenberg et al., 2002), transferring POLRMT and either or both of TFBM into MusTfam strain which is engineered to carry the mammalian mitochondrial promoters on its mtDNA would allow us to determine what genes are required for in vivo initiation of mammalian mitochondrial transcription as well as promoter sequences that are critical for initiating transcription in vivo. In addition, using this in vivo yeast model system, unknown factors that might be associated with the mammalian mitochondrial transcription could be screened by measuring promoter activities from the HSP and LSP. Furthermore different isoforms of Tfam that are either expressed in a broad range of tissues or in a tissue-specific manner such as testis-specific isoforms in mice and humans may play an important role in the concerted regulation of mitochondrial genomes during mitochondrial biogenesis (Bruno et al., 2007; Larsson et al., 1997; Tominaga et al., 1993). It has been reported that shorter transcript isoforms generated by alternative splicing may function as negative regulators (Foulkes and Sassone-Corsi, 1992). Some of the isoforms lack the ability to bind to their target sequence and other isoforms lack affinity to other transcriptional factors (Tominaga et al., 1993). Using the in vivo yeast model system, involvement of different transcript isoforms such as Tfamiso in the mitochondrial transcription initiation and regulation could be investigated by introducing necessary components into MusTfamiso strain because the transcript isoform can support maintenance and expression of yeast mitochondrial genome.
In conclusion, the mouse Tfam and its transcript isoform (Tfamiso) proteins can functionally replace the yeast mt-nucleoid protein Abf2p and support mtDNA maintenance and mitochondrial biogenesis in yeast. The mtDNA-supporting activity of mouse Tfam and Tfamiso was comparable to that of yeast Abf2p when the cells were subjected to a condition that required mitochondrial respiration for growth. These results demonstrate that mitochondrial HMG nucleoid proteins, including human TFAM, mouse Tfam and yeast Abf2p, are structurally and evolutionary conserved in their mtDNA-binding and -organizing activities under selective pressure in yeast mitochondria.
Supplementary Material
Acknowledgments
This work was supported by NIH grant NS052612, the Minnesota Medical Foundation, the Academic Health Center and the Institute of Human Genetics of the University of Minnesota to MDK and the National Research Foundation of Korea Grant (2011 0001262) funded by the Korean Government. We would like to thank Drs. Thomas D. Fox and Kathleen Conklin for the gift of the yeast strains.
Abbreviations
- mt
mitochondrial
- mtDNA
mitochondrial DNA
- Tfam
mitochondrial transcription factor A
- ABF2
ARS-binding factor 2
- HMG
high mobility group
- PCR
polymerase chain reaction
- GFP
green fluorescent protein
- FOA
5-fluoroorotic acid
- HSP
heavy strand promoter
- LSP
light strand promoter
- POLRMT
mitochondrial RNA polymerase
- TFBM
mitochondrial transcription factor B
Appendix A. Supplementary data
Supplementary data related to this article can be found online.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam TI, Kanki T, Muta T, Ukaji K, Abe Y, Nakayama H, Takio K, Hamasaki N, Kang D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003;31:1640–1645. doi: 10.1093/nar/gkg251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin-Cayuela J, Gustafsson CM. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem Sci. 2007;32:111–117. doi: 10.1016/j.tibs.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Biswas TK. Usage of non-canonical promoter sequence by the yeast mitochondrial RNA polymerase. Gene. 1998;212:305–314. doi: 10.1016/s0378-1119(98)00133-4. [DOI] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. doi: 10.1016/j.molcel.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Bonnefoy N, Fox TD. Genetic transformation of Saccharomyces cerevisiae mitochondria. Methods Enzymol. 2002;350:97–111. doi: 10.1016/s0076-6879(02)50958-7. [DOI] [PubMed] [Google Scholar]
- Bruno S, De Virgilio C, Gadaleta G. The smaller isoform of the mitochondrial transcription factor A has a role in the mitochondrial transcription. Ital J Biochem. 2007;56:315–318. [PubMed] [Google Scholar]
- Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- Clayton DA. Transcription and replication of mitochondrial DNA. Hum Reprod. 2000;15(Suppl 2):11–17. doi: 10.1093/humrep/15.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci USA. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi DJ, Shadel GS, Clayton DA. Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J Mol Biol. 1995;249:11–28. doi: 10.1006/jmbi.1995.9889. [DOI] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci USA. 1991;88:7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF, Stillman B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J Biol Chem. 1992;267:3368–3374. [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Dunstan HM, Green-Willms NS, Fox TD. In vivo analysis of Saccharomyces cerevisiae COX2 mRNA 5 ′-untranslated leader functions in mitochondrial translation initiation and translational activation. Genetics. 1997;147:87–100. doi: 10.1093/genetics/147.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet. 2002;31:289–294. doi: 10.1038/ng909. [DOI] [PubMed] [Google Scholar]
- Foulkes NS, Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992;68:411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Fox TD, Sanford JC, McMullin TW. Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proc Natl Acad Sci USA. 1988;85:7288–7292. doi: 10.1073/pnas.85.19.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- Jacobs HT. Making mitochondrial mutants. Trends Genet. 2001;17:653–660. doi: 10.1016/s0168-9525(01)02480-5. [DOI] [PubMed] [Google Scholar]
- Johnston SA, Anziano PQ, Shark K, Sanford JC, Butow RA. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988;240:1538–1541. doi: 10.1126/science.2836954. [DOI] [PubMed] [Google Scholar]
- Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann N Y Acad Sci. 2005;1042:101–108. doi: 10.1196/annals.1338.010. [DOI] [PubMed] [Google Scholar]
- Kanki T, Ohgaki K, Gaspari M, Gustafsson CM, Fukuoh A, Sasaki N, Hamasaki N, Kang D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004;24:9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LR, Megraw TL, Chae CB. Essential role of the HMG domain in the function of yeast mitochondrial histone HM: functional complementation of HM by the nuclear nonhistone protein NHP6A. Proc Natl Acad Sci USA. 1993;90:5598–5602. doi: 10.1073/pnas.90.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman D, Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993;15:539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- Larsson NG, Garman JD, Oldfors A, Barsh GS, Clayton DA. A single mouse gene encodes the mitochondrial transcription factor A and a testis-specific nuclear HMG-box protein. Nat Genet. 1996;13:296–302. doi: 10.1038/ng0796-296. [DOI] [PubMed] [Google Scholar]
- Larsson NG, Oldfors A, Garman JD, Barsh GS, Clayton DA. Down-regulation of mitochondrial transcription factor A during spermatogenesis in humans. Hum Mol Genet. 1997;6:185–191. doi: 10.1093/hmg/6.2.185. [DOI] [PubMed] [Google Scholar]
- Marra M, Hillier L, Kucaba T, Martin J, Beck C, Wylie T, Underwood K, Steptoe M, Theising B, Allen M, Bowers Y, Person B, Swaller T, Gibbons M, Pape D, Harvey N, Schurk R, Ritter E, Kohn S, Shin T, Jackson Y, Cardenas M, McCann R, Waterston R, Wilson R. The WashU-NCI Mouse EST Project 1999. 1999. Unpublished. [Google Scholar]
- Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Megraw TL, Chae CB. Functional complementarity between the HMG1-like yeast mitochondrial histone HM and the bacterial histone-like protein HU. J Biol Chem. 1993;268:12758–12763. [PubMed] [Google Scholar]
- Miyakawa I, Kanayama M, Fujita Y, Sato H. Morphology and protein composition of the mitochondrial nucleoids in yeast cells lacking Abf2p, a high mobility group protein. J Gen Appl Microbiol. 2010;56:455–464. doi: 10.2323/jgam.56.455. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991;252:965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- Parisi MA, Xu B, Clayton DA. A human mitochondrial transcriptional activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol Cell Biol. 1993;13:1951–1961. doi: 10.1128/mcb.13.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham JL, Dudley AM, Mason TL. T7 RNA polymerase-dependent expression of COXII in yeast mitochondria. Mol Cell Biol. 1994;14:4643–4652. doi: 10.1128/mcb.14.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York, NY: 1989. [Google Scholar]
- Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999;283:1488–1493. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Schena M, Picard D, Yamamoto KR. Vectors for constitutive and inducible gene expression in yeast. Methods Enzymol. 1991;194:389–398. doi: 10.1016/0076-6879(91)94029-c. [DOI] [PubMed] [Google Scholar]
- Shadel GS. Yeast as a model for human mtDNA replication. Am J Hum Genet. 1999;65:1230–1237. doi: 10.1086/302630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel GS, Clayton DA. Mitochondrial transcription initiation. Variation and conservation. J Biol Chem. 1993;268:16083–16086. [PubMed] [Google Scholar]
- Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annu Rev Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- Shutt TE, Bestwick M, Shadel GS. The core human mitochondrial transcription initiation complex: It only takes two to tango. Transcription. 2011;2:55–59. doi: 10.4161/trns.2.2.14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt TE, Lodeiro MF, Cotney J, Cameron CE, Shadel GS. Core human mitochondrial transcription apparatus is a regulated two-component system in vitro. Proc Natl Acad Sci USA. 2010;107:12133–12138. doi: 10.1073/pnas.0910581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia RA, Carrol S, Kalifa L, Hochmuth C, Sia EA. Loss of the mitochondrial nucleoid protein, Abf2p, destabilizes repetitive DNA in the yeast mitochondrial genome. Genetics. 2009;181:331–334. doi: 10.1534/genetics.108.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Hayashi J, Kagawa Y, Ohta S. Smaller isoform of human mitochondrial transcription factor 1: its wide distribution and production by alternative splicing. Biochem Biophys Res Commun. 1993;194:544–551. doi: 10.1006/bbrc.1993.1854. [DOI] [PubMed] [Google Scholar]
- Wach A, Pick H, Philippsen P. Procedures for isolating yeast DNA for different purposes. IRL Press; Oxford: 1994. [Google Scholar]
- Williamson D. The curious history of yeast mitochondrial DNA. Nat Rev Genet. 2002;3:475–481. doi: 10.1038/nrg814. [DOI] [PubMed] [Google Scholar]
- Wong TS, Rajagopalan S, Freund SM, Rutherford TJ, Andreeva A, Townsley FM, Petrovich M, Fersht AR. Biophysical characterizations of human mitochondrial transcription factor A and its binding to tumor suppressor p53. Nucleic Acids Res. 2009;37:6765–6783. doi: 10.1093/nar/gkp750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YG, Koob MD. Efficient cloning and engineering of entire mitochondrial genomes in Escherichia coli and transfer into transcriptionally active mitochondria. Nucleic Acids Res. 2003;31:1407–1415. doi: 10.1093/nar/gkg228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenaya-Troitskaya O, Newman SM, Okamoto K, Perlman PS, Butow RA. Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics. 1998;148:1763–1776. doi: 10.1093/genetics/148.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.