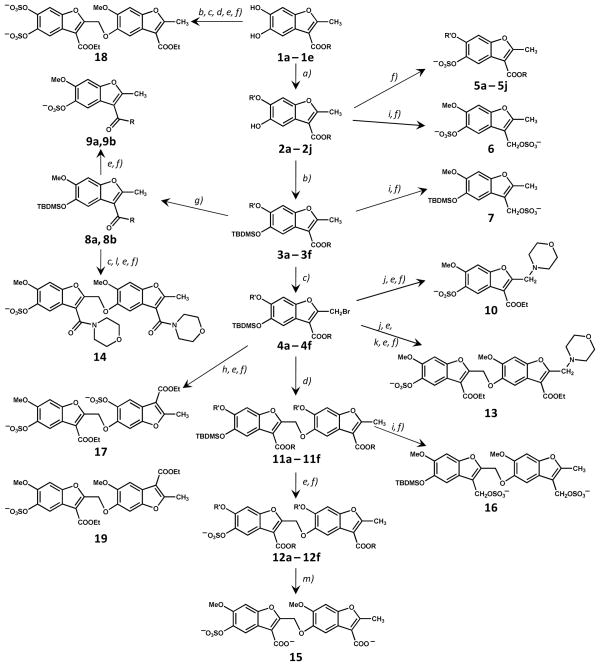
Figure 2.
Synthesis of a library of sulfated benzofurans as potential allosteric regulators of thrombin. The library consists of 15 monomers (5a – 5j, 6, 7, 9a, 9b, and 10) and 13 dimers (12a – 12f, 13–19). R and R′ groups are defined in Table 1. Synthesis of 19 is described in text and Supplementary Material. Reactions: a) Cs2CO3, alkyl or acyl halide, an. DMF; b) TBDMSCl, Imidazole, DMF; c) NBS, CCl4; d) Cs2CO3, 2; DMF; e) KF, CH3COOH, DMF; f) Et3N:SO3, CH3CN:DMF (4:1), MW; g) Me3Al, R-H, toluene; h) Cs2CO3, 1a, DMF; i) LAH, an. THF; j) Cs2CO3, morpholine, DMF; k) Cs2CO3, 4a, DMF; l) Cs2CO3, DMF, desilylated 8a; m) alc. LiOH. See text for details.