Abstract
Modulation of serotonin signaling by RNA editing of the serotonin 2C receptor (5HT2CR) may be relevant to affective disorder as serotonin functions regulate mood and behavior. Previously we observed enhanced endogenous behavioral despair in ADAR2 transgenic mice. Since the transcript of the 5HT2CR is a substrate of ADAR2, we hypothesized that perturbed ADAR2 equilibrium in the prefrontal cortex of ADAR2 transgenic mice alters the normal distribution of edited amino acid isoforms of the 5HT2CR and modifies the receptor function in downstream basal ERK signaling. We examined groups of naïve control and ADAR2 transgenic mice and found significantly increased ADAR2 expression, increased RNA editing at A, C, D and E sites and significantly altered normal distribution of edited amino acid isoforms of the 5HT2CR with increased proportions of VNV, VSV, VNI, INV and decreased INI amino acid isoforms of the 5HT2CR in ADAR2 transgenic mice. Localized serotonin levels (5-HT) were unchanged and perturbed ADAR2 equilibrium coincides with dysregulated edited amino acid isoforms of the 5HT2CR and reduced basal ERK signaling. These results altogether suggest that altered 5HT2CR function could be contributing to enhanced depression-like behavior of ADAR2 transgenic mice and further implicate ADAR2 as a contributing factor in cases of affective disorder.
Keywords: RNA editing, behavioral despair, ADAR2, ERK signal and 5HT2CR
INTRODUCTION
ADAR2 (ADARB1) is an RNA editing enzyme, which belongs to ADAR family of enzymes that catalyzes the conversion of adenosine to inosine (A-to-I) in pre-mRNA. ADAR2 auto edits its own transcript and regulates the ADAR2 protein expression (Feng et al., 2006, Rueter et al., 1999). A-to-I RNA editing occurs in several receptors and ion channels such as 5HT2CR, GluRB subunit of AMPA receptor, α3 subunit of the GABAA receptor and the KV1.1 potassium channel (Burnashev et al., 1992, Burns et al., 1997, Ohlson et al., 2007, Schmauss & Howe, 2002, Sommer et al., 1991). Amongst all these substrates the 5HT2CR is implicated in the pathophysiology of schizophrenia, bipolar disorder, anxiety and major depression (Frey et al., 2007, Gurevich et al., 2002b, Iwamoto & Kato, 2003, Lan et al., 2009, Niswender et al., 2001, Pilc et al., 2008, Sodhi et al., 2001).
The potential for dysregulated ADAR2 expression in aberrant RNA editing of the 5HT2CR in ADAR2 transgenic mice was examined as they display several endocrine and behavioral changes that has been associated with the 5HT2CR and affective disorder, such as elevated endogenous behavioral despair, anxiety-like behavior and a hyperactive hypothalamic pituitary-adrenal-axis (HPA) (Singh et al., 2009).
Reports from both humans and rodent models of depression suggest that abnormal RNA editing of the 5HT2CR is associated with depression (Dracheva et al., 2003, Fitzgerald et al., 1999, Gardiner & Du, 2006, Gurevich et al., 2002b, Iwamoto et al., 2005a, Schmauss, 2003, Yang et al., 2004), anxiety (Hackler et al., 2006) and Prader Willi-like syndrome (Morabito et al., 2010a). Both serotonin and antidepressants also alter RNA editing of the 5HT2CR (Fitzgerald et al., 1999, Gardiner & Du, 2006, Gurevich et al., 2002a, Schmauss & Howe, 2002, Tohda et al., 2006).
Central serotonergic neurotransmission is mediated by at least fourteen receptor subtypes, out of which, the 5HT2CR is the only one presently known to undergo A-to-I RNA editing, catalyzed by both ADAR1 and ADAR2 that generates 24 amino acid isoforms of the 5HT2CR, each with their distinct signaling properties (Burns et al., 1997, Wang et al., 2000, Werry et al., 2008a, Werry et al., 2008b). The altered amino acid sequence of 5HT2CR by RNA editing leads to reduced G protein coupling, reduced phospholipase C signaling (Burns et al., 1997) and altered phosphorylation of ERK signal (Labasque et al., 2010, Werry et al., 2008a, Werry et al., 2008b)
Altered 5-HT signaling and altered RNA editing of the 5HT2CR in the prefrontal cortex region is associated with depression (Englander et al., 2005, Iwamoto & Kato, 2003, Iwamoto et al., 2005b, Meltzer, 1989, Schmauss, 2003) and therefore RNA editing changes of the 5HT2CR may be relevant to depression-like behavior of ADAR2 transgenic mice. ERK signal participates in stress response and stress induces depressive-like behavior (Qi et al., 2008). Depression is ameliorated by brain derived neurotropic factor (BDNF) via activating the ERK pathway (Shirayama et al., 2002). Serotonin treatments induce extracellular signal-regulated kinase (ERK) phsophorylation (Lee et al., 2001) and cyclic AMP-responsive-element-binding protein (CREB) is a downstream target of ERK (Hardingham et al., 2001, Martinowich & Lu, 2008, Qi et al., 2008, Ying et al., 2002). RNA editing of the 5HT2CR is linked to ERK signaling and mood disorder (Dracheva et al., 2008a, Gurevich et al., 2002b, Iwamoto & Kato, 2003, Niswender et al., 2001, Werry et al., 2005, Werry et al., 2008a, Werry et al., 2008b). These results altogether suggest that ERK pathway is the major convergence point in all signal pathways of mood disorder and RNA editing could play a critical role in behaviors of ADAR2 transgenic mice.
In this study, the prefrontal cortex region from naïve control and ADAR2 transgenic mice was used to examine RNA editing changes of the 5HT2CR, compensatory changes in gene expression of ADAR family, expression changes in genes 5HT2CR, BDNF and CREB. We also examined the steady state protein levels of ADAR2, 5HT2CR and reduced function of the 5HT2CR on the downstream ERK signaling in control and ADAR2 transgenic mice.
MATERIALS AND METHODS
Animal husbandry
Animals were housed in individual plastic cages with cellular bedding. Rodent chow (7013 NIH-31 Modified diet), and tap water were available ad libitum for the duration of the experiments. All studies were conducted on ADAR2 transgenic or wild-type mice (e.g., control littermates) that were maintained on a B6D2F1 hybrid background by back-crossing to either a male or a female B6D2F1 mouse (Singh et al., 2007). The temperature in the mice colony room was maintained at 22°C. The light cycle was held at 12:12 h, with lights on at 0600 h. All procedures were approved and conducted in accordance with the University of Iowa Institutional Animal Care and Use Committee.
Genotyping
For genotype screening, sense and antisense primers corresponding to transgene were used to PCR amplify the transgene from the genomic DNA isolated from 10 μl of whole blood (obtained from tail snips) of control and transgenic mice using the RED Extract-N-Amp Blood PCR Kit (Sigma-Aldrich, St. Louis, MO) (Singh et al., 2007).
Animals used in this study
ADAR2 transgenic mouse misexpresses the active rat ADAR2b cDNA under the control of cytomegalovirus (CMV) promoter (Singh et al., 2007). Previously we observed that ADAR2 transgenic mice misexpressing the active ADAR2b enzyme displayed enhanced behavioral despair in both sexes without the use of chronic stress method that is typically used to induce depression-like behavior in rodent model of depression (Singh et al., 2009) and therefore ADAR2 transgenic mouse has been referred to as a rodent model of enhanced endogenous behavioral despair (Singh et al., 2009). Complex RNA editing changes from rodent models of depression have been previously reported using groups of 4 or more mice indicating that RNA editing changes in the 5HT2CR are homogenous in nature amongst animals to detect significant differences in a small group of animals (Englander et al., 2005, Iwamoto et al., 2005b). In this study we examined molecular changes in the prefrontal cortex region of two groups of naïve control and transgenic mice (n=4) and all our results of ADAR2 transgenic mice have been compared with their control littermates.
Quantitative real time PCR analysis of gene expression in the prefrontal cortex of control and transgenic mice
The prefrontal cortex region was isolated on a cryostat and total RNA was isolated from the prefrontal cortex by using TRIzol reagent (Invitrogen, Carlsbad, CA). One μg of total RNA from each animal was reverse transcribed with random hexamers (Roche Diagnostic Corp., Chicago, IL) into cDNA using SuperScript II Reverse Transcriptase (Invitrogen). The cDNA was subsequently used for quantitative PCR. Quantitative real time PCR experiments were carried out in a Bio-Rad iCycler (Bio-Rad Laboratories, Los Angeles, CA) using the fluorogenic intercalating dye SYBR green and gene specific primers of mouse or rat. For each gene of interest; CREB, BDNF, ADAR2, ADAR1 and ADAR3, the forward and reverse mouse specific primers were used (Table 1). Each 25 μl reaction contained 2 mM MgCl2, 1X PCR buffer, 0.65 μM each primer, 1.25 units Taq Polymerase, 5 μl cDNA and 2.5 μl SYBR green PCR master mix. PCR parameters were at an initial penetration at 95°C for 60s, followed by 35 cycles of 95°C for 20s, 60°C for 20s and 72°C for 20s. Relative gene expression was normalized with GAPDH expression. The cycle of amplification threshold (CT) was assessed, and the melting curve was used to verify the identity of the amplification products. The CT difference between the housekeeping gene GAPDH and the gene of interest for every sample (ΔCT) was determined.
Table 1.
Primers used for quantitative RT-PCR analysis of gene expression.
| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| BDNF | 5’GCATCTGTTGGGGAGACAAG3’ | TGGTCATCACTCTTCTCACCTG3’ |
| ADAR2 | 5’TCCTGCAGTGACAAGATAGCA3’ | 5’GGTTCCACGAAAATGCTGAG3’ |
| Transgene | 5’GCCCACCACCTACCACGAGTCC3’ | 5’CGGGGAGTTGGGCCTTGGATCCA3’ |
| ADAR1 | 5’GAGCAGTTGGGTTTCGCAG3’ | 5’AAACTGTTGGTCAGAGCATTGAAG3’ |
| ADAR3 | 5’GGGCATTTCTTCACTTCCTCT3’ | 5’GGTCCTCTTGATGCTTGCTC3’ |
| CREB | 5’CAGGCAGGTGTTTCACAGG | 5’GCATGTTCAGAGGGTTAGGG |
| 5HT2cR | 5’TGCTTAAAACTGAAGCAATAATGG3’ | 5’AGGCCAATTAGGTGCACAAG3’ |
Western blot analysis
Total cellular lysate from the prefrontal cortex was prepared using modified RIPA buffer [100 mM NaCl, 1% IGPAL CA 630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 7.6 with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 0.1% aprotinin; Sigma-Aldrich). Protein concentration was determined by BCA reagents (Thermo Scientific, Rockford, IL) and 20 μg of total protein from each mouse sample was resolved by denaturing polyacrylamide gel electrophoresis (10% SDS-PAGE) and transferred to a nitrocellulose membrane and rabbit anti-ADAR2-specific antiserum (1:1000 Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-5-HT2CR (1:500, Santa Cruz Biotechnology), rabbit anti-ERK (1:2000, cell signaling Technology Inc., Danvers, MA), rabbit anti-pERK (1:1000, cell signaling Technology Inc.), and rabbit-anti-β actin antiserum (1:2500 Sigma-Aldrich, St Luis, MO), was used as a control for normalizing loading for each sample (Dwivedi et al., 2001, Pandey et al., 2006, Singh et al., 2007, Werry et al., 2005). Anti-rabbit Alexa Fluor 680 (1:10,000 [vol/vol], Molecular Probes, Eugene, OR) was used as the secondary antibody. The secondary antibody was detected directly using an Odyssey infrared imaging system in accordance with the manufacturer's instructions (LI-COR Biosciences, Lincoln, NE) (Feng et al., 2006, Singh et al., 2007)
Immunohistochemistry
Control and ADAR2 transgenic mice were perfused intracardially first with 0.1 M phosphate buffer and then with 4% paraformaldehyde. Brains were extracted and post-fixed with 4% paraformaldehyde and cryoprotected in 30% sucrose. They were then cut on a freezing microtome at 40 μm and processed for ADAR2-immunoreactivity. Briefly, brain sections were soaked in 3% hydrogen peroxide and 10% methanol solution. The sections were rinsed in PBS, and then placed in normal goat serum solution. Sections were rinsed again with PBS before being placed in the primary antibody solution for 1 hr (rabbit anti-ADAR2 polyclonal IgG 1:5000, Santa Cruz Biotechnology). The brain sections were rinsed thoroughly with PBS, incubated in a secondary antibody overnight (anti-rabbit biotinylated IgG 1:200, Vector Laboratories, Burlingame, CA), and then rinsed again and soaked in PBS. The sections were reacted with Avidin–Biotin complex (Vector Laboratories), and then rinsed with PBS before a reaction with 0.02% diaminobenzidine solution. Hydrogen peroxide (0.01%) was used to initiate the reaction. Immediately following staining, the reaction was stopped by rinsing with PBS. Sections were mounted on slides and cover slipped with Permount (Fisher Scientific, Fairlawn, NJ) (Grippo et al., 2004).
RNA editing analysis of the prefrontal cortex of control and transgenic mice
PCR amplification of 5HT2CR RNA edited region
RNA isolated using the previous protocol was used to synthesize cDNAs. The cDNAs were subsequently used for further characterization of edited transcripts. PCR amplification of the 5HT2CR RNA edited region was performed using 1 mM concentration of each forward 5’TTTCAACTGCGTCCATCATGCACCT3’ and reverse primer 5’AACGAAGTTGGGGTCATTGAGCAC3’, 250 mM of dATP, dCTP, dGTP, and dTTP (dNTPs), 2 units of DNA polymerase and recommended buffer (Qiagen, Valencia, CA) in a total volume of 20 μl. Samples were amplified for 27 cycles; cycling conditions comprised 1 cycle at 96°C for 5 minutes followed by 27 cycles at 96°C for 15s, 50°C for 30s and 72°C for 30s, and finally 1 cycle of 72°C for 5 minutes in a thermal cycler (Bio-Rad Laboratories) resulting in 236bp amplicon. The 236bp PCR amplicon was gel purified using Qiaex II (Qiagen).
Characterization of RNA edited transcripts
The gel purified 236bp amplicon was subcloned in pGEM vector (Promega, Madison, WI). At least 45 individual colonies from each animal was picked and directly inoculated into the 30 μl PCR mixture containing; 0.2 μM sense oligonucleotide primer 5’ATATCGCTGGATCGGTATGTAG3’ and 0.2 μM antisense biotinylated primer 5’BIOTIN CGAATTGAAACGGCTATGCT3’, 1X Taq Gold buffer with 1.5 mM MgCl2 (PerkinElmer Life and Analytical Sciences, Shelton, CT), 0.75 units of Taq Gold (PerkinElmer) and 0.24 mM dNTPs and subjected to colony PCR. PCR was performed in a thermal cycler using the following conditions: 96°C for 5 minutes, then amplified for 30 cycles each consisting of 20s at 96°C, 30s at 58°C, and 20s at 72°C in a thermal cycler (Bio-Rad Laboratories). Five μl of the amplified single product (67bp) were electrophoresed on a 3% agarose gel to verify a single 67bp amplicon. The remaining 25 μl PCR product was subjected to high throughput pyrosequencing following the Biotage ABI based paradigm (Qiagen, Valencia, CA) (Hackler et al., 2006, Singh et al., 2007, Sodhi et al., 2005).
HPLC Methods for Biogenic amines
Tissue Extraction
The prefrontal cortex region was homogenized, using a tissue dismembrator, in 100-750 ul of 0.1M TCA, which contains 10-2 M sodium acetate, 10-4 M EDTA, 5ng/ml isoproterenol (as internal standard) and 10.5 % methanol (pH 3.8). Samples were spun in a micro centrifuge at 10000 g for 20 minutes. The supernatant was removed and stored at –80°C. The pellet was saved for protein analysis. Supernatant was then thawed and spun for 20 minutes. Samples of the supernatant were then analyzed for biogenic monoamines by HPLC method.
Biogenic amines
Biogenic amines were determined by a specific HPLC assay utilizing an Antec Decade II (oxidation: 0.5) electrochemical detector operated at 33° C. Twenty μl samples of the supernatant were injected using a Water 717+ auto sampler onto a Phenomenex Nucleosil (5u, 100A) C18 HPLC column (150 × 4.60 mm). Biogenic amines were eluted with a mobile phase consisting of 89.5% 0.1M TCA, 10-2 M sodium acetate, 10-4 M EDTA and 10.5 % methanol (pH 3.8). Solvent was delivered at 0.6 ml/min using a Waters 515 HPLC pump. Using this HPLC solvent the following biogenic amines elute in the following order: Noradrenaline, Adrenaline, DOPAC, Dopamine, 5-HIAA, HVA, 5-HT, and 3-MT. HPLC control and data acquisition were managed by Enpower 2 software (Vanderbilt Neurogenomics core lab, Vanderbilt University).
Protein
Total protein concentrations of prefrontal cortex extracts were determined using BCA Protein Assay Kit purchase from Pierce Chemical Company (Rockford, IL). The frozen pellets were allowed to thaw and reconstituted in a volume of 0.5 N HCl that equals that previously used for tissue homogenization. On hundred μl of this solution was combined with 2 ml of color reagent and allowed to develop for 2 hours. A BSA standard curve was run at the same time spanning the concentration range of 20-2000 μg/ml. Absorbance's of standard and samples were measured at 562 nm.
Statistical analyses
For quantitative real time PCR analysis, statistical analyses were performed on relative fold-changes determined by the ΔΔCT method and student t-tests were used to evaluate the significant difference of ΔΔCT values between transgenic (n=4) and control mice (n=4).
To test the effect of ADAR2 on the editing frequency of 5HT2CR in each of the 5 sites within the prefrontal cortex, logistic regression fitted by the method of generalized estimating equations (GEE) was used. The logistic regression model included as independent variables genotype (control mice vs. ADAR2 transgenic mice). The GEE method was used in fitting the logistic regression model to account for the correlation of site editing between clones from the same animal. The same analysis was performed to test for differences in amino acid isoform expression between ADAR2 transgenic and control mice. Independence of neighboring sites in transgenic and control mice was tested using the Pearson chi-square test. Independence of neighboring sites in transgenic and control mice was also tested using logistic regression fitted by the GEE method. This analysis was used for all neighboring site pairs except for A and B sites where there were zero cell counts for which computations for fitting a logistic model cannot be performed. For the A-B site pair, Pearson's Chi-square and Cochran-Mantel-Haenszel Chi-square were used.
RESULTS
Altered behavioral despair without altered serotonin levels in ADAR2 transgenic mice
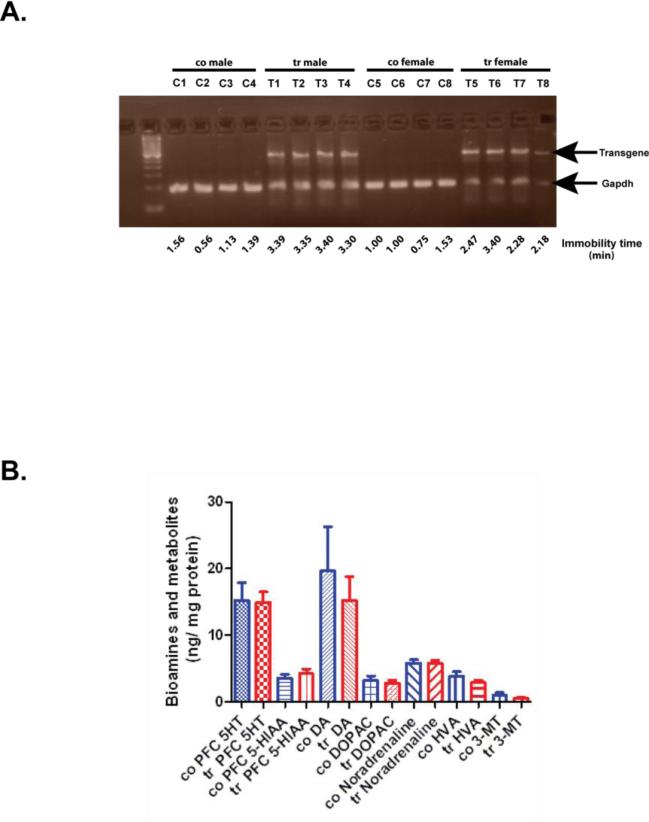
ADAR2 transgenic mice misexpress ADAR2 cDNA under the control CMV promoter and display enhanced behavioral despair without any experimental manipulation. Therefore termed as endogenous behavioral despair (Singh et al., 2009). In this study we determined the transgene expression from the prefrontal cortex tissue of previous studied ADAR2-Tg mice with enhanced endogenous behavioral despair (Singh et al., 2009). RT-PCR on RNA samples and gel electrophoresis of PCR products confirmed that the transgene was expressed in the prefrontal cortex of ADAR2 transgenic mice (Fig. 1a).
Figure 1. Transgene expression and bioamine levels in the prefrontal cortex of control and ADAR2 transgenic mice.
A) RT-PCR analysis on 1.8% agarose gel showing the RT-PCR product of transgene and GAPDH from the prefrontal cortex of control (n=8) and transgenic mice (n=8) from previous study (Singh et al., 2009) that displayed enhanced behavioral despair as determined by their immobility time shown below the gel picture. B) Mean ± SEM biogenic amine levels (ng/mg protein) from the prefrontal cortex of control (n=8) and ADAR2 transgenic mice (n=8).
HPLC analysis of biogenic amines showed no changes in serotonin, dopamine and noradrenaline levels in the prefrontal cortex of ADAR2 transgenic mice when compared to their control littermates (p>0.05, Fig 1b). These results suggest that behavioral despair in ADAR2 transgenic mice is independent of serotonin levels.
Increased ADAR2 mRNA in the prefrontal cortex of ADAR2 transgenic mice
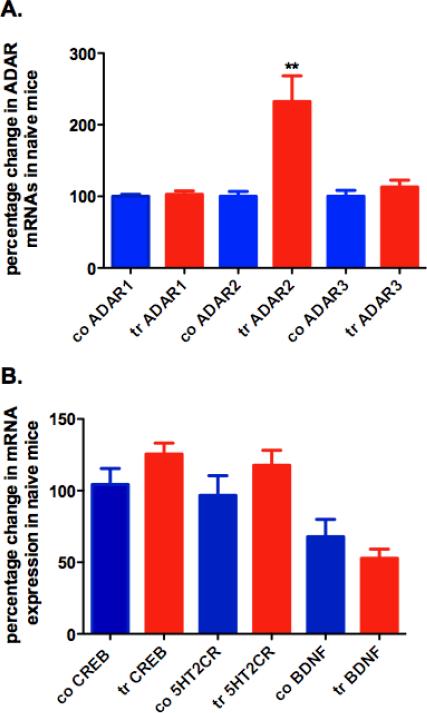
The total ADAR2 mRNA expression (mouse and rat) was significantly increased in the prefrontal cortex of ADAR2 transgenic mice when compared to control littermates (P<0.001, Fig. 2A).
Figure 2. Percentage change in gene expression in the prefrontal cortex of control and ADAR2 transgenic mice.
(a) Gene expression of ADAR family in the prefrontal cortex of control and transgenic mice. (b) Gene expressions of 5HT2CR, BDNF and CREB in control (n = 4) and transgenic mice (n = 4) (co, control, tr, transgenic, mean ± SEM, **, P < 0.001).
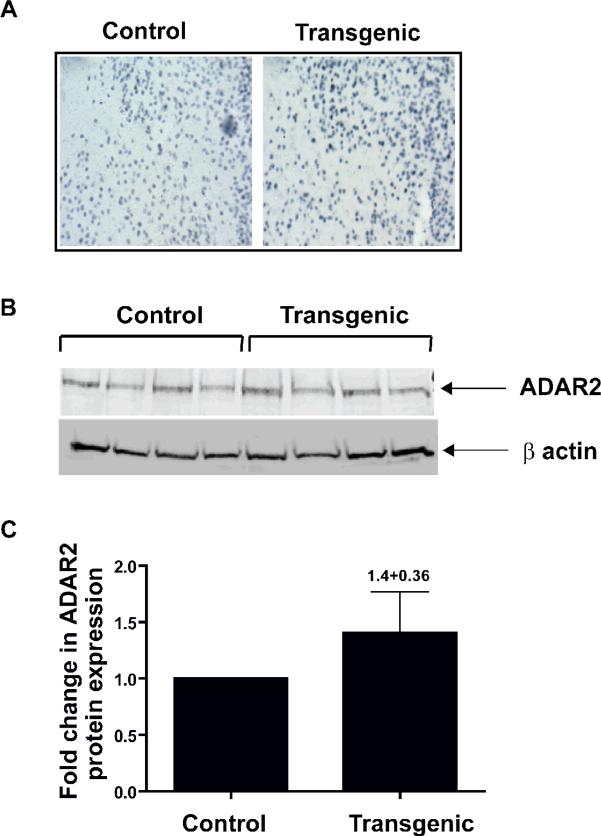
Qualitative immunohistochemistry showed increased ADAR2 protein expression in the prefrontal cortex of ADAR2 transgenic mice when compared to control littermates (Fig. 3A).
Figure 3. ADAR2 protein expression in the prefrontal cortex of control and transgenic mice.
A) A representative picture of immunohistochemistry analysis of ADAR2 expression in the prefrontal cortex of control and transgenic mice. B) Western analysis of ADAR2 protein expression from control (n=4) and transgenic mice (n=4). C) Histogram showing the fold changes in ADAR2 protein expression relative to control littermates (mean ± SEM 1.4±0.36).
Quantitative western analysis showed increased ADAR2 protein expression by 1.4±0.36 fold more in the prefrontal cortex of ADAR2 transgenic mice compared to control littermates (Fig. 3 B &C).
Gene expression analysis showed no changes in ADAR1 and ADAR3 mRNA expression as well as no changes in BDNF, CREB and 5HT2CR mRNA expressions when compared to their control littermates (Fig. 2A and B).
5HT2CR protein expression is unchanged in the prefrontal cortex of ADAR2 transgenic mice
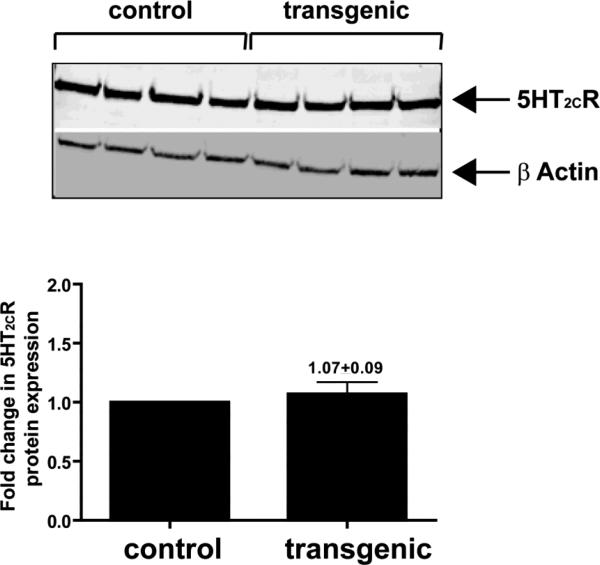
Western blot analysis showed no change in 5HT2CR protein expression in the prefrontal cortex of ADAR2 transgenic mice when compared to their control littermates (Fig.4A and B).
Figure 4. 5HT2CR protein expression from the prefrontal cortex of control and ADAR2 transgenic mice.
Western analysis of total cellular 5HT2CR protein expression in control (n=4) and transgenic mice (n=4) and histogram showing the fold change in 5HT2CR protein expression in ADAR2 transgenic mice relative to control littermates (mean±SEM, 1.07±0.09).
Distinct RNA editing changes of 5HT2CR pre-mRNA in the prefrontal cortex of ADAR2 transgenic mice
Pyrosequencing analysis of 67bp PCR amplicon showed a significant increased editing at A (p<0.002), C (p<0.0009), D (p<0.0001) and E (p<0.042) sites in ADAR2 transgenic mice when compared to their control littermates (Table 2). The clones from ADAR2 transgenic mice were more likely to exhibit 5HT2CR mRNA editing at these sites compared to control mice with odds ratio of 2.08 (95% CI: 1.32, 3.28) in edited site A, 1.60 (1.21, 2.11) in site C, 2.40 (2.20, 2.62) in site D and 7.85 (1.08, 57.09) in site E. The variation in the editing levels present in 5HT2CR transcripts was addressed also by determining total number of inosine residues. ADAR2 transgenic mice showed a significantly higher proportion of 5HT2CR transcripts with 3 or more inosine residues (P<0.0001; odds ratio: 1.97) and 4 or more inosine residues when compared to control mice (p<0.0001; odds ratio: 1.54; Table 3).
Table 2. Quantification of RNA editing at 5 modifiable sites of the 5HT2cR in control and transgenic mice.
The percentage of editing at each site was calculated from the number of clones sequenced.
| Editing site | Probability (95% CI) | Tr vs. Control | ||
|---|---|---|---|---|
| Tr | Control | Odds Ratio (95% CI) | p-value | |
| A | 91.0% (86.7%, 94.0%) | 82.9% (81.0%, 84.7%) | 2.08 (1.32, 3.28) | 0.002 |
| B | 93.9% (92.0%, 95.3%) | 94.6% (91.8%, 96.4%) | 0.88 (0.52, 1.48) | 0.630 |
| C | 20.2% (16.2%, 25.0%) | 13.7% (13.3%, 14.1%) | 1.60 (1.21, 2.11) | 0.0009 |
| D | 67.6% (66.0%, 69.0%) | 46.5% (45.2%, 47.8%) | 2.40 (2.20, 2.62) | 0.0001 |
| E | 2.8% (1.1%, 7.1%) | 0.4% (0.1%, 2.0%) | 7.85 (1.08, 57.09) | 0.042 |
| No editing | 0.5% (0.1%, 3.0%) | 5.1% (3.3%, 7.8%) | 0.10 (0.02, 0.61) | 0.123 |
Table 3. Number of inosine residues in the 5HT2cR transcript of control and transgenic mice.
The table represents the proportion of transcripts edited at any combination from 1 to 4 sites. The number of inosine residues was scored from individual clones and the proportion is represented as percentage of total clones sequenced.
| Number of Inosine Residue | Probability (95% CI) | Tr vs. Control | ||
|---|---|---|---|---|
| Tr | Control | Odds Ratio (95% CI) | p-value | |
| ≥4 | 15.7% (13.5%, 18.3%) | 10.8% (10.2%, 11.4%) | 1.54 (1.28, 1.87) | 0.0001 |
| ≥3 | 65.7% (65.4%, 66.0%) | 49.4% (48.2%, 50.5%) | 1.97 (1.88, 2.06) | 0.0001 |
Altered RNA editing site (independence from neighboring sites) in ADAR2 transgenic mice
All 5 sites that are modifiable are closely spaced within the 5HT2CR transcript. The possibility of one site editing and influencing the other or independent regardless of editing status was examined.
Linkage at A and B sites
Editing at A and B sites were not independent in both control and ADAR2 transgenic mice. The probability of A site editing was significantly higher when the B site was edited in both control and transgenic mice [Table 4. B (+) vs. (B-) Fisher's exact test p<0.0001] with a greater probability of A site editing seen in the transgenic group compared to control (Chi-square=11.47; p<0.0007). When the A site was not edited, the probability of editing at the B site was significantly lower in transgenic mice when compared to control mice (transgenic, 31.2% vs. control, 68.1%; Chi-square=6.68; p<0.010).
Table 4. RNA editing sites in control and transgenic mice (independence from neighboring sites).
Independence of neighboring sites in transgenic and control mice was tested using the Pearson chi-square test. Independence of neighboring sites in transgenic and control mice was also tested using logistic regression fitted by the GEE method. This analysis was used for all neighboring site pairs except for A and B sites where there were zero cell counts for which computations for fitting a logistic model cannot be performed. For the A-B site pair, Pearson's Chi-square and Cochran-Mantel-Haenszel Chi-square were used if editing at one site influenced the observed frequency of editing at adjacent sites.
| Site | Adjacent Site | Probability Site+ (when adjacent site is + or -) | Tr vs. Control p-value | |
|---|---|---|---|---|
| Tr | Control | |||
| A+ | B(+) | 97.1% | 87.7% | 0.0007 |
| B(-) | 0% (n=11) | 0% (n=15) | -- | |
| B(+) vs. B(-) | P<0.0001 | P<0.0001 | ||
| B+ | A (+) | 100% | 100% | -- |
| A (-) | 31.2% | 68.1% | 0.010 | |
| A (+) vs. A(-) | P<0.0001 | P<0.0001 | ||
| B+ | E(+) | 100% (n=5) | 100% (n=1) | -- |
| E(-) | 93.8% | 94.5% | 0.731 | |
| E(+) vs. E(-) | P=1.0 | -- | ||
| E+ | B(+) | 2.9% | 0.4% | 0.038 |
| B(-) | 0% (n=11) | 0% (n=15) | -- | |
| B(+) vs. B(-) | P=1.0 | P=1.0 | ||
| E+ | C(+) | 8.3% | 0% | 0.115 |
| C(-) | 1.4% | 0.4% | 0.560 | |
| C(+) vs. C(-) | P=0.05 | P=1.0 | ||
| C+ | E(+) | 60% (n=5) | 0% (n=1) | -- |
| E(-) | 18.8% | 13.5% | 0.134 | |
| C(+) vs. c(-) | P=0.05 | -- | ||
| C+ | D(+) | 23.0% | 22.6% | 0.945 |
| D(-) | 13.6% | 6.0% | 0.090 | |
| D(+) vs. D(-) | P=0.149 | P<0.0001 | ||
| D+ | C(+) | 77.8% | 75.7% | 0.832 |
| C(-) | 64.8% | 40.3% | 0.0001 | |
| C(+) VS. C(-) | P=0.149 | P<0.0001 | ||
Linkage at E and B sites
No evidence of linkage was observed between the E and B sites in either transgenic or control mice. However, in ADAR2 transgenic mice, we did find that when the B site was edited, there was significantly greater E site editing compared to their control littermates (Fisher's exact test, p<0.038).
Linkage at E and C sites
A linkage was observed at E and C site editing in ADAR2 transgenic mice (Fisher's exact test p<0.05) with a significantly higher probability of E site editing when the C site was edited. However, similar linkage at E and C site editing was not observed in control mice.
Linkage at C and D sites
In control mice, the probability of D site editing was significantly greater in the adjacent C edited site versus the C non-edited site (Chi square =16.15, p<0.0001; 75.7% vs. 40.3%). These results indicate that there is a linkage at C and D sites observed in control mice. Similar pattern was observed in transgenic mice with smaller differences in the probability of D site editing (or C site editing) in the context of edited versus non-edited adjacent site. This showed a significant interaction effect between genotype and editing in the neighboring site (Wald Chi square=16.42, p<0.0001), indicating that the degree of linkage is significantly favorable in control mice compared to ADAR2 transgenic mice.
Altered amino acid isoforms of the 5HT2CR in the prefrontal cortex of naïve transgenic mice
The clones from the ADAR2 transgenic mice had a significantly higher proportion of sequences coding for the 5HT2CR isoforms with VNV, VSV, INV and other grouped minor amino acid isoform expression (VNV: transgenic = 45.3% vs. control = 34.5%; P<0.0001; VSV: transgenic=13.3% vs. control 10.2%, p<0.0001; INV: transgenic=5.0% vs. 0.4%, p<0.003; grouped minor amino acid isoforms: transgenic = 5.5% vs. 0.4%, p<0.007) when compared to control mice (Table 5). The odds ratio of VNV isoforms in transgenic mice relative to control mice was 1.57 (confidence interval = 95%; transgenic = 1.36, control = 1.82), and VSV isoform odds ratio 1.35 (confidence interval=95%; transgenic= 1.20, control =1.51), INV isoform odds ratio 14.34 (confidence interval=95%; transgenic =2.53, control =81.2) and grouped minor isoform odds ratio 16.02 (confidence interval =95%; transgenic = 2.16, control = 118.6). The ADAR2 transgenic mice expressed a lower proportion of the VNI and INI isoforms compared to control mice (VNI: transgenic = 26% vs. control = 34.5%, p<0.0001; INI: transgenic = 0.6% vs. control =5.1%, p<0.012). The odds ratio of VNI and INI isoforms in transgenic mice relative to control were (VNI: 0.66, tr= 0.58, co = 0.76; INI: 0.10. tr =0.02, co = 0.61).
Table 5. Predicted amino acid isoforms of the 5HT2cR in control and transgenic mice.
cDNAs encoding the predicted amino acid isoforms were scored from individual clones and the frequency of amino acid isoforms were calculated from the total clones sequenced.
| Edited Amino Acid Isoform | Probability (95% CI) | Tr vs. Control | ||
|---|---|---|---|---|
| Tr | Control | Odds Ratio | p-value | |
| Valine Asparagine Valine (Valine Asparagine Valin) | 45.3% (42.2%, 48.4%) | 34.5% (32.8%, 36.3%) | 1.57 (1.36, 1.82) | 0.0001 |
| Valine Serine Valine (Valin Serine Valin) | 13.3% (12.5%, 14.1%) | 10.2% (9.4%, 11.0%) | 1.35 (1.20, 1.51) | 0.0001 |
| Valine Asparagine Isoleucine (Valine Asparagine Isoleucine) | 26.0% (23.6%, 28.5%) | 34.5% (33.3%, 35.9%) | 0.66 (0.58, 0.76) | 0.0001 |
| Isoleucine Asparagine Valine (Isoleucine Asparagine Valine) | 5.0% (4.3%, 5.8%) | 0.4% (0.06%, 2.0%) | 14.34 (2.53, 81.2) | 0.003 |
| Isoleucine Asparagine Isoleucine (Isoleucine Asparagine Isoleucine) | 0.6% (0.1%, 3.0%) | 5.1% (3.4%, 7.6%) | 0.10 (0.02, 0.61) | 0.012 |
| (Valine Serine Isoleucine) (VSI) | 3.9% (2.9%, 5.1%) | 3.2% (2.6%, 4.0%) | 1.19 (0.83, 1.70) | 0.342 |
| Valine Aspartic acid Valine (VDV), Valine Aspartic acid Isoleucine (VDI), Valine Glycine Isoleucine (VGI), Valine Glycine Valine (VGV), Isoleucine Serine Valine (ISV), Methionine Serine Valine (Msv), Methionine Asparagine Isoleucine (MNI), Methionine Asparagine Valine (MNV) | 5.5% (2.1%, 13.9%) | 0.4% (0.06%, 2.0%) | 16.02 (2.16, 118.6) | 0.007 |
Altered ERK signal in the prefrontal cortex of naïve ADAR2 transgenic mice
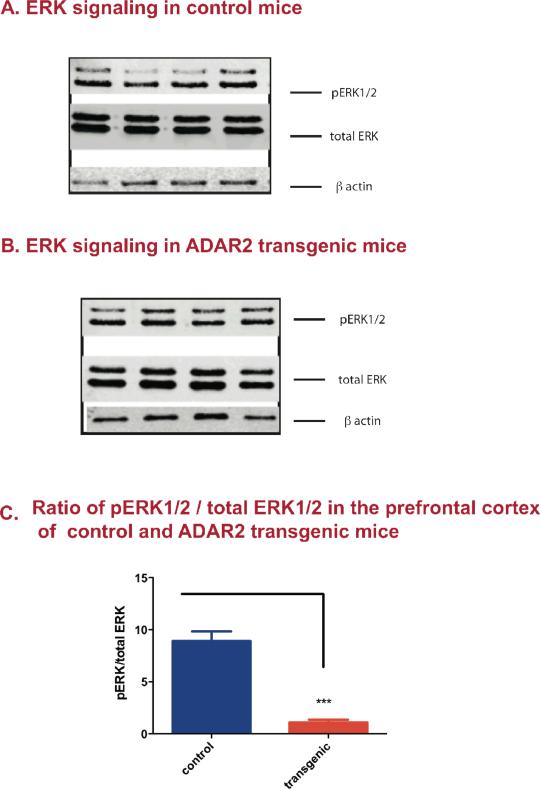
Western analysis showed significant reduction of basal ERK phosphorylation in the prefrontal cortex of ADAR2 transgenic mice when compared to their control littermates (p<0.0001, Fig. 5c).
Figure 5. Altered phosphorylated ERK1/2 (pERK1/2) signaling in the prefrontal cortex of control and ADAR2 transgenic mice.
Western blot analyses were done on pERK1/2) and total ERK1/2, that were normalized to β actin. To determine the amount of pERK1/2, the ratio of pERK1/2: total ERK1/2 was calculated for each animal and the graph represents the mean ±SEM ratio of pERK1/2: total ERK in control and ADAR2 transgenic mice (n=4/group). A) Western blot analysis of pERK1/2, total ERK and β actin protein expression in control mice B) Western blot analysis of pERK1/2, total ERK and β actin protein expression in ADAR2 transgenic mice. C) Mean ±SEM ratio of pERK1/2/total ERK1/2 in control and ADAR2 transgenic mice is represented the histogram. Student t-test analysis showed that there was a significant decreased basal pERK1/2 in ADAR2 transgenic mice when compared to their control littermates (n=4/group, mean± SEM, ***=p<0.0001).
DISCUSSION
This is the first study in a rodent model of enhanced endogenous behavioral despair, ADAR2 transgenic mice where ADAR2 misexpression in the prefrontal cortex results in; 1) perturbed ADAR2 equilibrium, 2) significant changes in editing and altered distribution of edited amino acid isoforms of the 5HT2CR, 3) no changes in 5HT2CR, BDNF, CREB, ADAR1 and ADAR3 mRNA expression, 4) no changes in localized levels of biogenic amines levels and 5) reduced basal ERK phosphorylation.
RNA editing of the 5HT2CR affects many facets of 5HT2CR signaling (Berg et al., 2008, Labasque et al., 2010, Werry et al., 2008a, Werry et al., 2008b) due to the repertoire of edited amino acid isoforms. The five sites that are edited in the pre-mRNA are conserved between human and rodents, but they differ in their levels and patterns of editing (Gardiner & Du, 2006). It is this combinatorial editing at 5 modifiable sites, that results in 32 mRNA variants and 24 amino acid isoforms from a single gene locus.
The significant changes in RNA editing of the 5HT2CR in the prefrontal cortex of ADAR2 transgenic mice is most likely a consequence of perturbed ADAR2 equilibrium as expression of other ADARs were unaltered (Fig. 2A) and in addition no changes were detected in local 5-HT levels (Fig. 1b) which have the potential to alter the RNA editing status of the 5HT2CR (Gurevich et al., 2002a). At the protein level ADAR2 was found to be only slightly elevated despite the significant increase in total ADAR2 mRNA expression (Fig. 3A, B and C). This could be due to negative feedback loop that regulates the expression of endogenous ADAR2 protein (Feng et al., 2006, Rueter et al., 1999). Therefore the protein expression difference that is observed is probably due to dysregulated rat ADAR2 expression. It is interesting to note this slight change in ADAR2 protein equilibrium coincides with significant RNA editing changes and edited amino acid isoforms of the 5HT2CR highlighting the importance of ADAR homeostasis. Further, recent report of increased ADAR1 expression from the cortical region of depressed patients with suicide (Simmons et al., 2010) and reports of altered RNA editing of the 5HT2CR in depression (Dracheva et al., 2008b, Gurevich et al., 2002b) highlights the importance of normal levels of ADARs in depression.High throughput pyrosequencing from 466 clones, determined the editing status of 5HT2CR mRNA in the prefrontal cortex of control and ADAR2 transgenic mice. ADAR2 transgenic mice showed significant increased editing at A (P<0.002), C (P<0.0009), D (P<0.0001) and E sites (P<0.042) compared to their control littermates (Table 2). In rodent model of depression, chronic mouse stress is generally employed to induce depression-like behavior and this kind of manipulation coincides with RNA editing changes in the 5HT2CR (Englander et al., 2005, Iwamoto et al., 2005b). A similar observation of increased A, C and E site editing were observed in ADAR2 transgenic mice but without any experimental manipulation. Studies in rodent models of depression and humans show increased editing at C and E sites reduces the G-protein coupling of the 5HT2CR and correlates with depression (Gurevich et al., 2002a, Wang et al., 2000). Thus increased editing C and E sites may have reduced 5-HT signaling in ADAR2 transgenic mice. Since ADAR2 transgenic mice display enhanced endogenous behavior despair, we examined other gene players in depression such as BDNF and CREB as they are influenced by serotonin, stress and antidepressants (Martinowich & Lu, 2008, Qi et al., 2008). There were no changes in BDNF and CREB expression when compared to control littermates thereby suggesting that compromised 5-HT signaling by the modified 5HT2CR may be a main contributing factor to affective disorder in ADAR2 mice.
Two members of ADAR family ADAR1 and ADAR2 show preferences for the editing sites in the 5HT2CR transcripts with A and B sites preference for ADAR1 and at C and D sites preference by ADAR2 (Beal et al., 2007, Du et al., 2006, Gardiner & Du, 2006, Hartner et al., 2004, Higuchi et al., 2000, Lehmann & Bass, 1999, Lehmann & Bass, 2000, Maas et al., 1999, Wang et al., 2004, Wong et al., 2001), We examined if perturbed ADAR2 equilibrium modified and influenced RNA editing at one site over the other in the context of being either edited or non-edited in ADAR2 transgenic and control mice (Table 4). RNA editing at one site can also disrupt the RNA duplex secondary structure to the extent that ADAR2 cannot properly bind or function on other sites. Overexpression of ADAR2 protein in vitro results in competition at the A site editing of ADAR1 (Chen et al., 2000, Singh et al., 2007). Thus, potential for perturbed ADAR2 equilibrium interfering with ADAR1's preferred A and B sites and other site editing were examined. We observed several changes in context of neighboring edited sites influencing the other site editing in ADAR2 transgenic mice; there was a significant increased editing at A site when B site was edited, increased editing at E site when either B or C site were edited and D site increased when C site was edited. However, specificity of D site was lost in ADAR2 transgenic mice. These results suggest certain secondary structures influences the order of editing at the neighboring site and that when perturbed ADAR2 equilibrium is present it leads to promiscuous editing at A, C and E sites resulting in aberrant editing of the 5HT2CR in ADAR2 transgenic mice. Similar increased editing changes have been observed in other rodent models of depression (Dracheva et al., 2003, Gurevich et al., 2002a, Gurevich et al., 2002b, Iwamoto & Kato, 2003). It is interesting to note that the recurring theme in all studies show dynamic editing changes at E and C sites in depression and response to drugs and environment stimuli. Further studies are needed to see if behavioral changes correspond to the dynamics of editing in response to environmental stimuli and drug treatments in ADAR2 transgenic mice.
RNA editing at five sites lead to presence of inosines within a short stretch of the transcript that can disrupt the secondary structure of mRNA and destabilize translation. Many inosine can alter base pairing efficiency of tRNAs within the inosine containing codons, lead to nonsense mediated decays and favor nuclear retention of inosine containing RNAs. As a result translational efficiency can be altered (Kozak, 1980, Lim, 1995, Scadden, 2007, Scadden, 2008, Seal et al., 1989). Despite significant increased 3 or more inosines in the 5HT2CR transcript of ADAR2 transgenic mice, the total cellular 5HT2CR protein expression was unaltered suggesting that translational efficiency of the 5HT2CR was unchanged in ADAR2 transgenic mice (Fig. 4a and b and table 3).
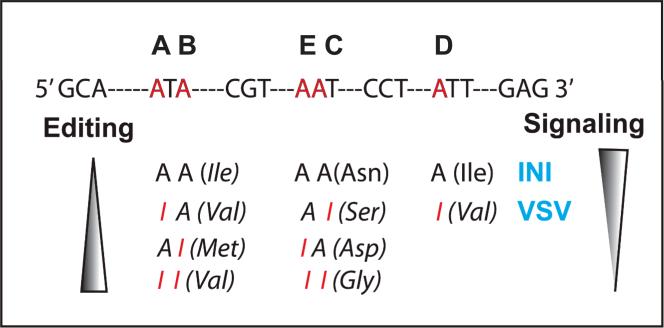
Amino acid isoforms of the 5HT2CR generated by RNA editing display distinct biochemical and pharmacological properties of the 5HT2CR (Berg et al., 2001, Fitzgerald et al., 1999, Labasque et al., 2010, Marion et al., 2004, Mcgrew et al., 2004, Price & Sanders-Bush, 2000, Price et al., 2001, Werry et al., 2008a, Werry et al., 2008b). The general observation is that the unedited INI isoform is the constitutive isoform, has the highest G protein coupling and phospholipase C signaling and that more editing leads to reduced G protein coupling and phospholipase C signaling (Fig. 6). Modified receptor isoforms have been correlated with behavior (Du et al., 2006, Morabito et al., 2010b) and also differences in editing patterns corresponds to behavioral responses produced by stressful environments (Englander et al., 2005, Gardiner & Du, 2006, Iwamoto et al., 2005b).
Figure 6. RNA editing and 5HT2CR isoforms.
Genomic sequence and five modifiable A residues (A-E) in the transcripts of 5HT2CR (top), and predicted amino acid changes associated with RNA editing of the A residues in the 5HT2CR transcript showing their direction of signaling strength as a function of the amino acid isoforms generated by combinatorial editing (bottom).
We observed significant increase in VNV, VSV, VNI, INV and minor grouped amino acid isoforms and a significant decreased INI amino acid isoform in ADAR2 transgenic mice compared to controls. ERK signaling is coupled to the 5HT2CR and editing alters the ERK signaling (Berg et al., 2008, Berg et al., 2001, Labasque et al., 2010, Werry et al., 2008a, Werry et al., 2008b). We therefore examined ERK phosphorylation status in the prefrontal cortex of control and ADAR2 transgenic mice. We observed reduced ERK phosphorylation indicating reduced basal signaling. This observation is consistent with our findings of altered amino acid isoforms of the 5HT2CR. It is interesting to note that significant reduced constitutive INI isoform coincides with reduced phosphorylation of basal ERK1/2 thus suggesting that 5-HT signaling may be compromised in ADAR2 transgenic mice. However, further studies are required to confirm a direct relationship between pERK1/2 signaling pathways and the edited changes of the 5HT2CR and enhanced behavioral despair (Labasque et al., 2010, Singh et al., 2009).
We cannot exclude the possibility that other possible edited isoforms were present but went undetected in our study due to lack of sensitivity by pyrosequencing method and may also play functionally relevant roles in affective disorder. Recently, deep sequencing methods have been used to detect all predicted 24 amino acid isoforms of the 5HT2CR (Abbas et al., 2010, Morabito et al., 2010b). Deep sequencing may be useful in the future to detect all the predicted 24 amino acid isoforms in ADAR2 transgenic mice to more thoroughly define which groups of edited isoforms play a role in affective disorder of ADAR2 transgenic mice. Thereafter genetic manipulation to expressing the disease-associated ratio of key edited isoforms in the prefrontal cortex to recapitulate the depression-like behavior of ADAR2 transgenic mice.
Further studies using antidepressants that alter RNA editing of the 5HT2CR in ADAR2 transgenic mice will attempt to attenuate the depression-like behavior and will further strengthen the role of edited 5HT2CR in affective disorder. Finally we cannot exclude the possibility that other ADAR2 modified substrates may be involved in affective disorder of ADAR2 transgenic mice. Future studies on transcriptome wide analysis using deep sequencing on Solexa platform should identify other substrates with potential contribution to the behavioral phenotypes of ADAR2 transgenic mice. Nevertheless, our findings here suggest that perturbed ADAR2 expression substantially changes the distribution of 5HT2CR isoforms which supports the concept that 5HT2CR RNA editing is a player in depression.
Acknowledgements
This research was mainly supported by Nation Institute of Mental Health (MS) MH082234-02 and in some part by National Heart, Lung, and Blood Institute HL-14388, National Institute of Diabetes, Digestive and Kidney Diseases DK-66086 (AKJ). We would like to thank Dr. Colleen Stein for critically reviewing the manuscript.
REFERENCES
- Abbas AI, Urban DJ, Jensen NH, Farrell MS, Kroeze WK, Mieczkowski P, Wang Z, Roth BL. Assessing serotonin receptor mRNA editing frequency by a novel ultra high-throughput sequencing method. Nucleic Acids Res. 2010;38:e118. doi: 10.1093/nar/gkq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal PA, Maydanovych O, Pokharel S. The chemistry and biology of RNA editing by adenosine deaminases. Nucleic Acids Symp Ser (Oxf) 2007;51:83–84. doi: 10.1093/nass/nrm042. [DOI] [PubMed] [Google Scholar]
- Berg KA, Clarke WP, Cunningham KA, Spampinato U. Fine-tuning serotonin2c receptor function in the brain: molecular and functional implications. Neuropharmacology. 2008;55:969–976. doi: 10.1016/j.neuropharm.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Cropper JD, Niswender CM, Sanders-Bush E, Emeson RB, Clarke WP. RNA-editing of the 5-HT(2C) receptor alters agonist-receptor-effector coupling specificity. Br J Pharmacol. 2001;134:386–392. doi: 10.1038/sj.bjp.0704255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. Rna. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Chin B, Haroutunian V. Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport. 2008a;19:379–382. doi: 10.1097/WNR.0b013e3282f556d2. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Elhakem SL, Marcus SM, Siever LJ, McGurk SR, Haroutunian V. RNA editing and alternative splicing of human serotonin 2C receptor in schizophrenia. J Neurochem. 2003;87:1402–1412. doi: 10.1046/j.1471-4159.2003.02115.x. [DOI] [PubMed] [Google Scholar]
- Dracheva S, Patel N, Woo DA, Marcus SM, Siever LJ, Haroutunian V. Increased serotonin 2C receptor mRNA editing: a possible risk factor for suicide. Mol Psychiatry. 2008b;13:1001–1010. doi: 10.1038/sj.mp.4002081. [DOI] [PubMed] [Google Scholar]
- Du Y, Davisson MT, Kafadar K, Gardiner K. A-to-I pre-mRNA editing of the serotonin 2C receptor: comparisons among inbred mouse strains. Gene. 2006;382:39–46. doi: 10.1016/j.gene.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol Cell Biol. 2006;26:480–488. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Iyer G, Conklin DS, Krause CM, Marshall A, Patterson JP, Tran DP, Jonak GJ, Hartig PR. Messenger RNA editing of the human serotonin 5-HT2C receptor. Neuropsychopharmacology. 1999;21:82S–90S. doi: 10.1016/S0893-133X(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- Gardiner K, Du Y. A-to-I editing of the 5HT2C receptor and behaviour. Brief Funct Genomic Proteomic. 2006;5:37–42. doi: 10.1093/bfgp/ell006. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Na ES, Johnson RF, Beltz TG, Johnson AK. Sucrose ingestion elicits reduced Fos expression in the nucleus accumbens of anhedonic rats. Brain Res. 2004;1019:259–264. doi: 10.1016/j.brainres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002a;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002b;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Airey DC, Shannon CC, Sodhi MS, Sanders-Bush E. 5-HT(2C) receptor RNA editing in the amygdala of C57BL/6J, DBA/2J, and BALB/cJ mice. Neurosci Res. 2006;55:96–104. doi: 10.1016/j.neures.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem. 2004;279:4894–4902. doi: 10.1074/jbc.M311347200. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Estimating RNA editing efficiency of five editing sites in the serotonin 2C receptor by pyrosequencing. Rna. 2005a;11:1596–1603. doi: 10.1261/rna.2114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Kato T. RNA editing of serotonin 2C receptor in human postmortem brains of major mental disorders. Neurosci Lett. 2003;346:169–172. doi: 10.1016/s0304-3940(03)00608-6. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Nakatani N, Bundo M, Yoshikawa T, Kato T. Altered RNA editing of serotonin 2C receptor in a rat model of depression. Neurosci Res. 2005b;53:69–76. doi: 10.1016/j.neures.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the “scanning model” for initiation of protein synthesis in eucaryotes. Cell. 1980;22:7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- Labasque M, Meffre J, Carrat G, Becamel C, Bockaert J, Marin P. Constitutive activity of Serotonin2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants. Mol Pharmacol. 2010;78:818–826. doi: 10.1124/mol.110.066035. [DOI] [PubMed] [Google Scholar]
- Lan MJ, McLoughlin GA, Griffin JL, Tsang TM, Huang JT, Yuan P, Manji H, Holmes E, Bahn S. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2009;14:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, Kim CJ, Chung JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:610, 725–618. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. The importance of internal loops within RNA substrates of ADAR1. J Mol Biol. 1999;291:1–13. doi: 10.1006/jmbi.1999.2914. [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry. 2000;39:12875–12884. doi: 10.1021/bi001383g. [DOI] [PubMed] [Google Scholar]
- Lim VI. Analysis of action of the wobble adenine on codon reading within the ribosome. J Mol Biol. 1995;252:277–282. doi: 10.1006/jmbi.1995.0494. [DOI] [PubMed] [Google Scholar]
- Maas S, Gerber AP, Rich A. Identification and characterization of a human tRNA-specific adenosine deaminase related to the ADAR family of pre-mRNA editing enzymes. Proc Natl Acad Sci U S A. 1999;96:8895–8900. doi: 10.1073/pnas.96.16.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- McGrew L, Price RD, Hackler E, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor disrupts transactivation of the small G-protein RhoA. Mol Pharmacol. 2004;65:252–256. doi: 10.1124/mol.65.1.252. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Serotonergic dysfunction in depression. Br J Psychiatry Suppl. 1989;8:25–31. [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis. 2010a;39:169–180. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito MV, Ulbricht RJ, O'Neil RT, Airey DC, Lu P, Zhang B, Wang L, Emeson RB. High-throughput multiplexed transcript analysis yields enhanced resolution of 5-hydroxytryptamine 2C receptor mRNA editing profiles. Mol Pharmacol. 2010b;77:895–902. doi: 10.1124/mol.109.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor: alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABA(A) receptor subunit alpha3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M. Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res. 2006;31:167–176. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Price RD, Sanders-Bush E. RNA editing of the human serotonin 5-HT(2C) receptor delays agonist-stimulated calcium release. Mol Pharmacol. 2000;58:859–862. doi: 10.1124/mol.58.4.859. [DOI] [PubMed] [Google Scholar]
- Price RD, Weiner DM, Chang MS, Sanders-Bush E. RNA editing of the human serotonin 5-HT2C receptor alters receptor-mediated activation of G13 protein. J Biol Chem. 2001;276:44663–44668. doi: 10.1074/jbc.M106745200. [DOI] [PubMed] [Google Scholar]
- Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–285. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- Scadden AD. Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol Cell. 2007;28:491–500. doi: 10.1016/j.molcel.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden AD. Gene expression is reduced in trans by inosine-containing dsRNA. Biochem Soc Trans. 2008;36:534–536. doi: 10.1042/BST0360534. [DOI] [PubMed] [Google Scholar]
- Schmauss C. Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist. 2003;9:237–242. doi: 10.1177/1073858403253669. [DOI] [PubMed] [Google Scholar]
- Schmauss C, Howe JR. RNA editing of neurotransmitter receptors in the mammalian brain. Sci STKE. 2002;133:pe26. doi: 10.1126/stke.2002.133.pe26. [DOI] [PubMed] [Google Scholar]
- Seal SN, Schmidt A, Marcus A. Ribosome binding to inosine-substituted mRNAs in the absence of ATP and mRNA factors. J Biol Chem. 1989;264:7363–7368. [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M, Meador-Woodruff JH, Sodhi MS. Increased cortical expression of an RNA editing enzyme occurs in major depressive suicide victims. Neuroreport. 2010;21:993–997. doi: 10.1097/WNR.0b013e32833f11c3. [DOI] [PubMed] [Google Scholar]
- Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J Biol Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- Singh M, Zimmerman MB, Beltz TG, Johnson AK. Affect-related behaviors in mice misexpressing the RNA editing enzyme ADAR2. Physiol Behav. 2009;97:446–454. doi: 10.1016/j.physbeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi MS, Airey DC, Lambert W, Burnet PW, Harrison PJ, Sanders-Bush E. A rapid new assay to detect RNA editing reveals antipsychotic-induced changes in serotonin-2C transcripts. Mol Pharmacol. 2005;68:711–719. doi: 10.1124/mol.105.014134. [DOI] [PubMed] [Google Scholar]
- Sodhi MS, Burnet PW, Makoff AJ, Kerwin RW, Harrison PJ. RNA editing of the 5-HT(2C) receptor is reduced in schizophrenia. Mol Psychiatry. 2001;6:373–379. doi: 10.1038/sj.mp.4000920. [DOI] [PubMed] [Google Scholar]
- Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- Tohda M, Nomura M, Nomura Y. Molecular pathopharmacology of 5-HT2C receptors and the RNA editing in the brain. J Pharmacol Sci. 2006;100:427–432. doi: 10.1254/jphs.cpj06005x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- Wang Q, O'Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- Werry TD, Gregory KJ, Sexton PM, Christopoulos A. Characterization of serotonin 5-HT2C receptor signaling to extracellular signal-regulated kinases 1 and 2. J Neurochem. 2005;93:1603–1615. doi: 10.1111/j.1471-4159.2005.03161.x. [DOI] [PubMed] [Google Scholar]
- Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther. 2008a;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Werry TD, Stewart GD, Crouch MF, Watts A, Sexton PM, Christopoulos A. Pharmacology of 5HT(2C) receptor-mediated ERK1/2 phosphorylation: agonist-specific activation pathways and the impact of RNA editing. Biochem Pharmacol. 2008b;76:1276–1287. doi: 10.1016/j.bcp.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Wong SK, Sato S, Lazinski DW. Substrate recognition by ADAR1 and ADAR2. Rna. 2001;7:846–858. doi: 10.1017/s135583820101007x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wang Q, Kanes SJ, Murray JM, Nishikura K. Altered RNA editing of serotonin 5-HT2C receptor induced by interferon: implications for depression associated with cytokine therapy. Brain Res Mol Brain Res. 2004;124:70–78. doi: 10.1016/j.molbrainres.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]