Figure 2.
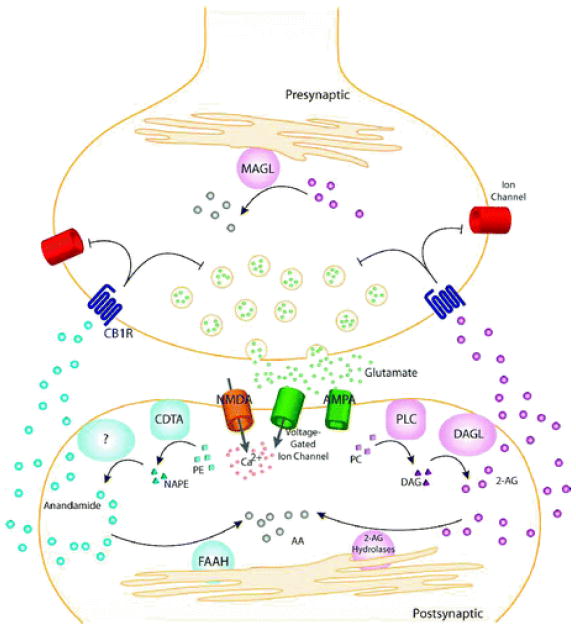
General model for endocannabinoid-based retrograde signaling. Upon release of neurotransmitter (e.g., glutamate), postsynaptic receptors (e.g., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl- d-aspartic acid (NMDA)) and voltage-gated ion channels are activated, allowing influx of Ca 2+ and on-demand endocannabinoid biosynthesis. Anandamide is synthesized from phospholipid precursors by a calcium-dependent transacylase (CDTA) and one or more other still uncharacterized enzymes. 2-Arachidonoylglycerol (2-AG) is synthesized from phospholipid precursors by phospholipase C (PLC) and diacylglycerol lipase (DAGL). Endocannabinoids then migrate from postsynaptic neurons to CB1 receptors (CB1R) located on presynaptic neurons. Once activated, CB1Rs couple through the G i/G o class of G-proteins to regulate ion channels and inhibit neurotransmitter release. The retrograde signaling of endocannabinoids is then terminated by degradative enzymes. Anandamide is hydrolyzed to arachidonic acid (AA) primarily by fatty acid amide hydrolase (FAAH), located in the postsynaptic neuron. 2-AG is hydrolyzed to AA primarily by monoacylglycerol lipase (MAGL) in the presynaptic neuron, though other 2-AG hydrolases may also participate in this process.
