Figure 4.
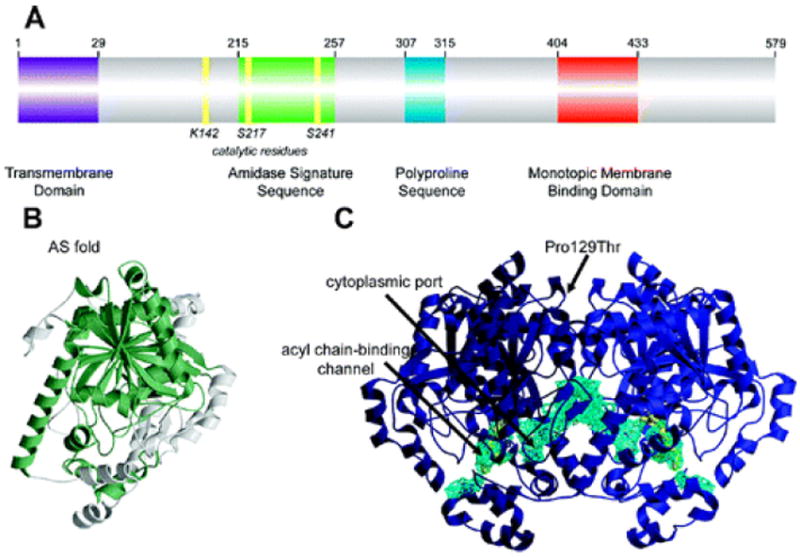
Structural features of FAAH: (A) Primary sequence analysis reveals a predicted NH 2-terminal transmembrane domain (purple), an amidase signature sequence rich in glycine and serine residues (green), a polyproline sequence predicted to interact with Homer and SH3 domain-containing proteins (blue), and a monotopic membrane binding domain that enables FAAH to bind the membrane even in the absence of the transmembrane domain (red). (B) An overlay of the known structures of amidase signature (AS) enzymes reveals a common “AS fold”, shown in green for the FAAH monomer. (C) Two channels in the FAAH X-ray crystal structure suggest possible routes for substrate binding (acyl chain-binding channel) and product release (cytoplasmic port). Structural studies also revealed that a common human single nucleotide polymorphism, which results in mutation of Pro129 to threonine, is located on the putative cytoplasmic face of FAAH.
