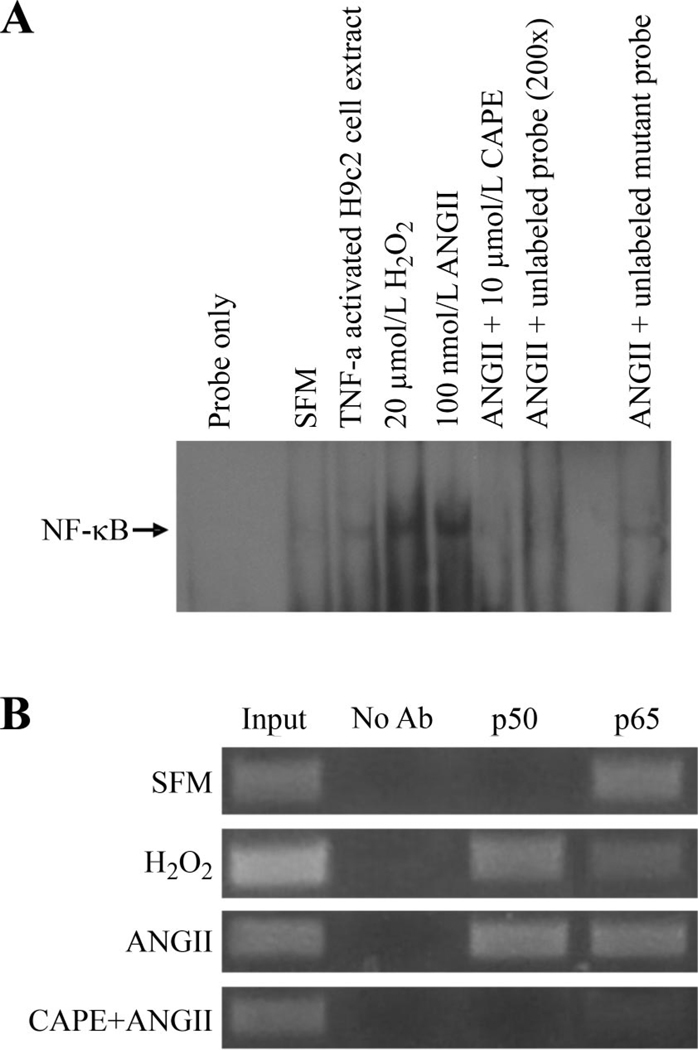
Fig. 6.
ANG II and H2O2 induce NF-κB binding to the scn5a promoter. A: electrophoretic mobility shift assays with nuclear extracts from H9c2 cardiomyocytes showed that under basal conditions (SFM), there was little binding of NF-κB to the scn5a promoter. H2O2 or ANG II exposure resulted in the NF-κB binding to the promoter. Tumor necrosis factor (TNF)-α-activated H9c2 cell nuclear extract (5 µg) was used as positive control. Caffeic acid phenethyl ester (CAPE, 10 µmol/l), a NF-κB inhibitor, prevented binding in response to H2O2. ANG II-induced NF-κB binding was inhibited by unlabeled probe but not mutant unlabeled probe. B: ANG II and H2O2 promote binding of the p50 subunit of NF-κB to the cardiac Na+ channel promoter. Chromosomal immunoprecipitation assay using primers specific for scn5a promoter shows that the p65 subunit of NF-κB appears to be constitutively bound to the channel promoter but that the p50 subunit binds in response to ANG II or H2O2. CAPE, an inhibitor of NF-κB, could prevent binding of both subunits to the channel promoter. Lanes 1 and 2 are positive and negative controls, respectively. The input DNA was diluted with 1:10.