Uterine myomas (leiomyomas) are an important problem in women's health. Leiomyoma treatment has evolved significantly from the late 19th century when the Chairman's address to the American Medical Association's section on Obstetrics and Gynecology argued that myomectomy was “so dangerous and difficult as not to be thought of except in desperate conditions.”1 Although many alternatives to hysterectomy are widely used, hysterectomy remains the mainstay of treatment in the United States where approximately 200,000 hysterectomies for leiomyomas are performed annually2 with only a recent decline noted in hysterectomy use.3,4
In contrast, over the same timeframe, treatment of ectopic pregnancies and endometriosis has evolved from a time when every affected woman required major surgery to one in which minimally invasive surgical approaches and medical treatments predominate. Why does hysterectomy continue to dominate treatment for leiomyomas?
A concrete impediment to innovation is the heterogeneity of disease in terms of size, location, growth trajectory, and symptomatology of leiomyomas. Symptomatic leiomyomas range from sizes small enough to not be palpable or visible by ultrasonography to ones large enough to distort a woman's abdominal contour mimicking pregnancy.
Having a single therapy such as hysterectomy obviates the complicated decision-making brought on by a disease with many presentations. Myomas are a common phenotype representing many genotypes and somatic mutations leading to different symptoms and growth patterns; this makes recommendations for treatment more complex and individual. An analogous situation would be if Crohn's disease, ulcerative colitis, and celiac disease were not individually characterized so that all gastrointestinal disease seemed to have an unpredictable course. Our goal as leiomyoma researchers is to understand the biology of this diversity and provide evidence to guide individualized treatment for the future.
Limitations in Our Knowledge
Most data on leiomyomas are still based on expert opinion. Although nearly 70–80% of women will have leiomyomas,5 most sources state that leiomyomas are symptomatic in approximately 25% of women. However, given the widespread use of steroidal agents for contraception, women with symptomatic leiomyomas may appear asymptomatic as a result of these hormonal treatments. Prevalence studies using ultrasonographic screening indicate that leiomyomas start at a young age and increase with age until menopause in nearly all populations studied.5–7 However, population studies have primarily been performed in Western cultures.
African American women are particularly affected by leiomyomas. They have myomas diagnosed at an earlier age, a higher incidence and prevalence of disease, evidence of more severe disease, and different patterns of myomas.5,6,8–10 African American women are three times more likely to have a hysterectomy for leiomyomas,11 yet the higher disease burden among African American women has yet to be explained. There is some evidence that different genes and genetic polymorphisms may underlie the severe phenotype of leiomyomas in African American women, including increases in aromatase, signal transduction genes, and transcription factors among African American women compared with whites.12,13
Lack of a standardized nomenclature and classification makes communication difficult in clinical care and research. Efforts at standardized definitions such as the European Society of Hysteroscopy's classification14 of submucous leiomyomas can aid communication but are not universally adopted. Instead of describing a 3-cm leiomyomas “distorting the uterine cavity,” the sonographer can more clearly describe a leiomyoma with no intramural extension (class 0), with less than 50% intramural extension (class I), or with 50% or more intramural extension (class II). The clinician then can move toward hysteroscopic myomectomy for the first (Fig. 1) and a levonorgestrel intrauterine system or endometrial ablation for the third in the woman with heavy menstrual bleeding.
Fig. 1.
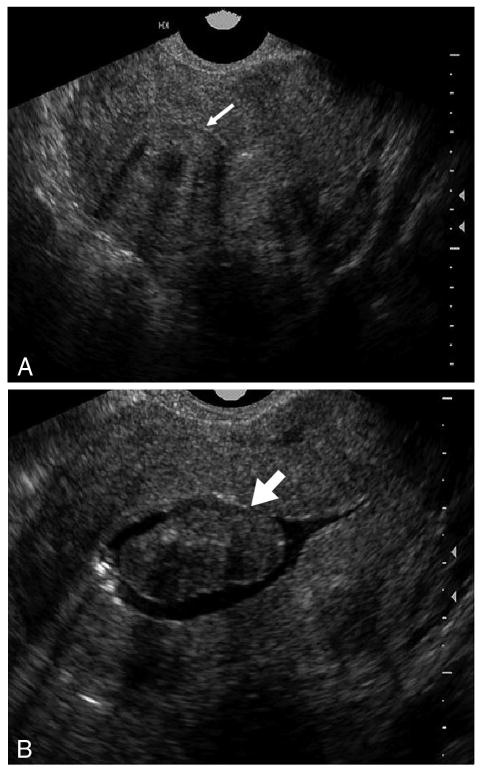
Class 0 submucosal leiomyoma. On transvaginal ultrasonography (A), a central myoma is seen, but the relationship to the endometrial cavity is unclear (thin arrow). With a sonohysterogram (B), the complete intracavitary extent is clarified (thick arrow).
Leiomyoma Growth
Given the high prevalence of leiomyomas, inhibiting growth or inducing regression appears to be the main opportunity for prevention. We now have data to suggest that growth and regression are highly variable among leiomyomas.15 On average, leiomyoma growth tends to be slow with a median of 9% change in volume in a 6-month period. However, leiomyoma growth and shrinkage ranged from −89% to +138% both within the same uterus and between different women.15
Likewise, during pregnancy, leiomyomas do not inevitably grow. In most studies, the majority of leiomyomas remained the same size.16,17 Additionally, spontaneous shrinking was found in nearly 80% of women within 6 months of delivery.18 Postpregnancy remodeling of the uterus may affect leiomyomas, creating natural therapy during the reproductive years and explanation of the protective effect of parity on leiomyoma risk.19–21
Growth rates also appear to be related to race. In the Fibroid Growth Study, white women had significantly lower leiomyoma growth rates as they approached menopause, whereas African American women's growth rates remained unchanged.15 Thus, although expectant management for a white woman in her mid-40s may be appropriate, it may be less effective for an African American woman.
These data add to previous studies that have documented that “rapid growth” in premenopausal women is not associated with an increased risk of malignancy. In a study of over 1,300 women, 0.23% of women who had surgery for leiomyomas had any type of uterine sarcoma and among the 371 women with surgery for a rapidly growing leiomyoma, 0.27% had a leiomyosarcoma.22 Thus, women should not undergo hysterectomy on the basis of rapid leiomyoma growth.23
Heterogeneity of Biology and Pathology
The clinical heterogeneity of leiomyomas is rooted in biologic differences. Leiomyoma development and growth has long been associated with gonadal steroid hormones and especially estrogen24 (Fig. 2). Both estrogen and progesterone play important roles in leiomyoma biology, yet selective steroidal modulation produces variable clinical outcomes. Aromatase inhibitors appear to be a promising therapy, but selective estrogen receptor modulator treatment has been more efficacious in animal models than in human studies.23,25 Blocking progesterone response therapeutically appears to be an effective strategy based on clinical data yet no progesterone receptor modulators have made it to clinical approval.26 Even control of steroid hormones has not provided a magic bullet for leiomyoma therapy.
Fig. 2.
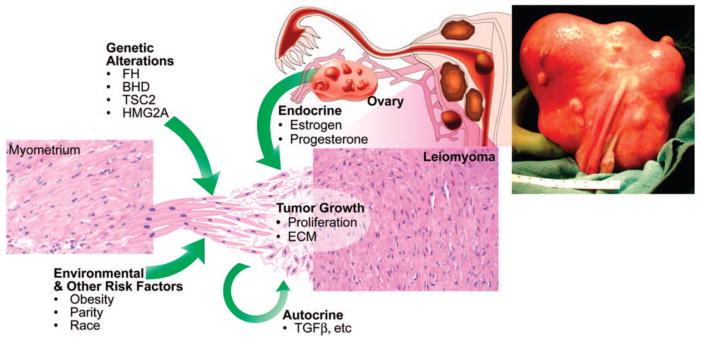
Etiology of uterine leiomyomas. Leiomyomas are heterogenous in their natural history and etiology. Hereditary defects in the FH, BHD, and TSC2 genes and somatic alterations affecting HMG2A genes contribute to the development of leiomyomas, as do risk factors such as obesity, parity, and race. Tumor growth occurs by an increase in tumor cell number and extracellular matrix production and is promoted by both endocrine and autocrine growth factors. Reprinted from Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science 2005;308:1589–92. Reprinted with permission from the American Association for the Advancement of Science.
Chromosomal or karyotypic abnormalities are found in approximately 40% of leiomyomas with several common rearrangements predominating.27 Specific karyotypes are associated with the size and uterine location of leiomyomas.28,29 Recent studies have also documented differences in gene profiles with specific karyotypes.30 There is hope that understanding karyotypic changes that occur in leiomyomas may lead to novel treatment options as they have with cancerous tumors such as chronic myelogenous leukemia.
Work is ongoing to identify novel susceptibility genes for leiomyomas through the Finding Genes for Fibroids Study using a genomewide scan and the Black Women's Health Study using admixture mapping. One leiomyoma gene, fumurate hydratase, causes a rare autosomal-dominant genetic syndrome (hereditary leiomyomatosis and renal cell cancer syndrome) characterized by skin leiomyomas and papillary renal cell carcinoma in addition to uterine leiomyomas and an increased risk of uterine sarcomas at a young age.27
A current hypothesis is that myometrial injury initiates a cascade of changes in growth factors resulting in cellular proliferation, decreased apoptosis, and increased extracellular matrix production.31,32 The transforming growth factor-β system is a key mediator in this system.33,34 This fibrosis of the tumors leads to its name “fibroids” and there has been a more recent push to refer to them in the literature as fibroids to stress the importance of extracellular matrix in these tumors.31 Angiogenic growth factor dysregulation, and particularly basic fibroblast growth factor, is also a key pathway in many leiomyomas and may be related to heavy menstrual bleeding.33
Microarray analyses have found that 25% of the genes expressed differentially between the myometrium and leiomyoma tissue are genes involved in transforming growth factor-β signaling and collagen or extracellular matrix production.35 These pathways allow for interesting points of intervention and opportunities for prevention. Current research involves retinoic acid,36 pirfenidone,37 components of green tea,38 and vitamin D.39 Targeting the initial “injury” such as reproductive tract infections and inflammation or even painful menses may prevent tumors as well, but there are little data on these issues.32,40,41
Ideal Candidates for Minimally Invasive Therapies
It is difficult to provide evidence-based recommendations regarding leiomyomas treatments because there is so little clinical research, particularly in U.S. populations or other areas with substantial racial diversity. In fact, the Agency for Healthcare Research and Quality states in their evidence-based review there is little evidence for our standard of care treatments, much less innovative treatments.42
Leiomyoma symptoms should be carefully elicited because this influences treatment choice. For women whose only symptom is heavy bleeding, there are several options after pregnancy, endometrial, and hormonal causes of bleeding have been ruled out. Hysteroscopic resection of type 0 and I submucosal leiomyomas associated with menorrhagia is straightforward.23 Larger submucosal leiomyomas may be amenable to shrinkage with gonadotropin-releasing hormone analogs to improve the chances of complete resection and maximize preoperative iron stores.
For heavy or prolonged menses in a woman without a submucous leiomyoma, a number of medical options exist, including steroidal therapies such as oral contraceptive pills and the levonorgestrel intrauterine system. Contraceptive patches and vaginal rings likely work in a similar fashion. However, research is indicated to see if increased local delivery of steroids to the uterus with vaginal devices has implications for leiomyomas. The levonorgestrel intrauterine system has been shown to work effectively in women with leiomyomas, although expulsion of the levonorgestrel intrauterine system may be more common.43 Recent Food and Drug Administration approval of tranexamic acid, an oral antifibrinolytic, for menorrhagia provides a new nonhormonal option in the United States that has been widely used elsewhere. Used only during the menstrual cycle, tranexamic acid has been associated with up to a 50% decrease in bleeding in women with menorrhagia, but there are few studies on women with leiomyomas. One study found no difference in blood loss with tranexamic acid therapy but had a sample size of 12 women with leiomyomas.44 Thrombotic risk was found to be similar to that of other menorrhagia treatments.45 There is retrospective evidence of increased necrosis in leiomyomas among tranexamic acid users.46
Endometrial ablation is a minimally invasive surgical option for menorrhagia when there is not a significant uterine cavity distortion and no desire for future pregnancy. Several devices are Food and Drug Administration-approved for use in the myomatous uterus, but most studies have been performed with minimally enlarged and distorted uteri. In a retrospective study, leiomyomas did not increase the failure rate of endometrial ablation, but a cavity measuring over 9 cm in depth increased chance of continued menses.47
For women with pressure symptoms attributable to myomas (bladder discomfort, constipation, back or pelvic pressure; Fig. 3) with or without menorrhagia, there are several alternatives to hysterectomy, including myomectomy, uterine artery embolization and magnetic resonance-guided focused ultrasonography. Advanced preoperative imaging, including magnetic resonance imaging (MRI) or sonohysterogram may help determine the best treatment for each patient. MRI (Fig. 4) with gadolinium may demonstrate leiomyoma devascularization which limits treatment by focused ultrasound or uterine artery embolization. MRI may also help identify lesions suspicious for sarcoma.48 Sonohysterograms may identify class 0 leiomyomas or other hysteroscopically resectable lesions.
Fig. 3.
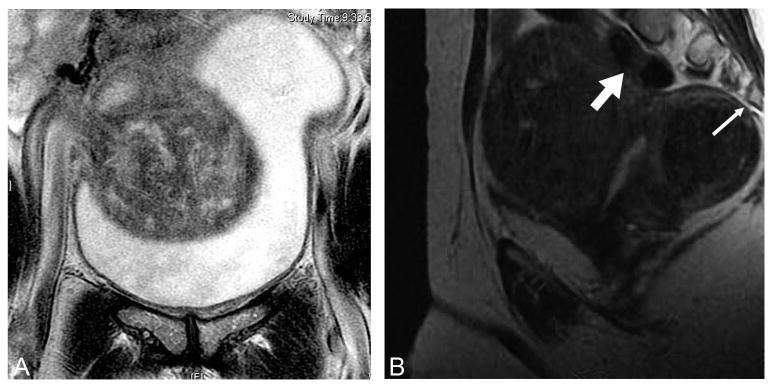
Magnetic resonance imaging (MRI) demonstrating leiomyoma compressing (A) the bladder and (B) the spine (thin arrow) and colon (thick arrow). Bulk symptoms from leiomyomas include decreased bladder capacity and outflow obstruction, constipation, back pain, and sciatica.
Fig. 4.
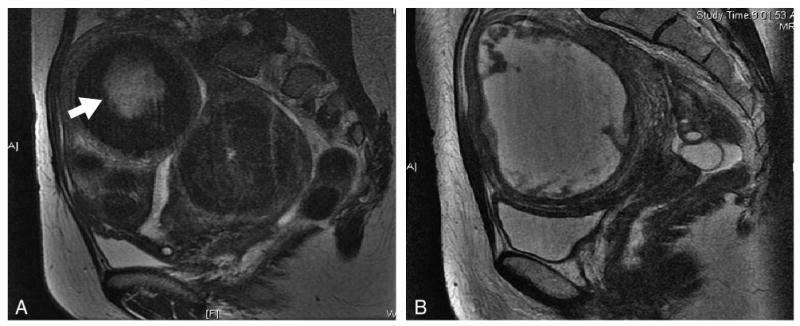
Degenerating leiomyomas seen on T2-weighted magnetic resonance imaging (MRI; A; arrow) and on T1-weighted MRI (B) with gadolinium contrast.
Myomectomy can increasingly be performed laparoscopically, with or without robotic assistance. Laparoscopic myomectomy reduces recovery time and is appropriate for subserosal and large intramural leiomyomas. Robotic assistance is associated with fewer intraoperative complications and lower blood loss than traditional laparoscopy in retrospective studies.49 It likely provides better uterine closure than laparoscopic suturing but data are limited.49 Open myomectomies may still be optimal for 1) a uterus with multiple intramural tumors that may not be easily identifiable or require multiple uterine incisions; 2) very large leiomyomas in which operative time is increased; and 3) uteri so large that an endoscopic approach is not possible.
Uterine artery embolization involves a short treatment using conscious sedation, an incision in the groin to access femoral vessels, and an overnight stay for pain control. Studies including randomized clinical trials in Europe document durable symptom control for 1 to 5 years with improvement in bleeding, pain, and bulk symptoms.50,51 Uterine artery embolization has comparable adverse event rates to surgery with decreased procedural pain. Women have a significantly faster return to work after uterine artery embolization, but some women go on to additional surgical intervention.50
Solitary leiomyomas more than 10 cm or multiple fibroids with a uterine volume consistent with a 20-week or greater gestation are considered relative contraindications to uterine artery embolization, but treatment has been successful.52 Prior surgeries and adhesions are not impediments for uterine artery embolization. Additionally, the ovarian impairment after uterine artery embolization may be an asset for perimenopausal women.53
Magnetic resonance-guided focused ultrasonography makes the transition from minimally invasive to noninvasive therapy; ultrasonographic energy goes through the abdominal wall and coagulates areas of the leiomyoma with real-time MRI monitoring providing control. Magnetic resonance-guided focused ultrasonography continues to grow in popularity because of high patient satisfaction and minimal side effects.54 Women go home the same day after a 3-hour treatment with some uteri requiring two treatments. Leiomyomas up to 10 cm in diameter or several medium-sized fibroids are routinely treated, and optimal results are achieved with complete treatment. Even with less than 20% leiomyoma volume treated (determined by nonperfused volume ratio), symptom severity scores significantly drop and remain low for 24 months.54
Because magnetic resonance-guided focused ultrasonography treats individual leiomyomas, there may be leiomyomas that cannot be successfully treated and rare locations that are inaccessible. Extensive abdominal wall scarring or failure to include surgical scars in treatment planning can lead to an increased risk of skin burns. Major contraindications are metal implants, defibrillators, or other contraindications to undergoing MRI.
One key advantage of minimally invasive treatments is a shorter recovery time. Women return to work 1 day to 2 weeks after most of these procedures compared with 6 weeks for abdominal hysterectomies. Studies document that disability costs are significant for leiomyomas and loss of work costs averaged over $700 per year per woman in one study.55,56 A National Institutes of Health-funded randomized controlled trial comparing uterine artery embolization with magnetic resonance-guided focused ultrasonography for leiomyomas is now underway, which will assess costs in the United States (NCT00995878, clinicaltrials.gov).
For some women, hysterectomy will still remain a viable choice. With all other treatments, new leiomyomas may form (commonly but incorrectly called recurrence) and thus hysterectomy provides definitive management. Moreover, for women at high risk of cervical, uterine, ovarian, or breast cancer, removal of the uterus, even with ovarian conservation, decreases cancer risk. However, hysterectomy has significant short-term morbidity and potentially long-term morbidity, including pelvic prolapse and adhesion formation, and should not be undertaken without discussion of risks and benefits.
Special Issues for Women Desiring Fertility
When women develop leiomyomas before childbearing, treatments that preserve optimal uterine function are critical. In previous generations when women had their children early and had few contraceptive options, uterine-sparing options were used less. The decision regarding fertility is more complicated than often assumed. There is a range of answers from no desire for fertility to women who are actively pursuing pregnancy. However, there is a large group of women who may accept some fertility-impairing side effects of treatments, which preserve the uterus.
For women with leiomyomas desiring fertility, there are several areas of concern. Will leiomyomas increase the risk of infertility, miscarriage, or pregnancy complications? Alternatively, what are the fertility-impairing implications of treatment?
For women with type 0 or I submucous leiomyomas 5 cm or less in mean diameter, the decision-making is straightforward. This is the subgroup of leiomyomas in which the evidence is strongest for fertility impairment and hysteroscopic myomectomy has few fertility-inhibiting side effects.23,57
Myomectomy remains the standard for treatment of leiomyomas that allows preservation of fertility but itself carries significant morbidity related to pregnancy, including adhesion formation, anemia, rare risk of hysterectomy, and, for laparoscopic repairs, possible increased risk in uterine rupture. Thus, risks and benefits of alternatives should be discussed and patient autonomy respected, especially for women with increased surgical risks.
For uterine artery embolization, there does appear to be an age-related risk of ovarian impairment.58 However, data from a randomized clinical trial comparing uterine artery embolization with hysterectomy in Europe showed that both groups had evidence of diminished ovarian reserve, although the trend was toward a greater decline at 1 year for women undergoing uterine artery embolization.59 Additionally, in a large cohort study, there was approximately a 12% risk of placental abnormalities after uterine artery embolization, all in nulliparous women.58 Nonetheless, many women have had healthy pregnancies after uterine artery embolization.
For focused ultrasonography, there are less data, but initial reports of reproductive outcomes are encouraging. Fifty-four pregnancies in 51 women have been recently reported.60 Live birth occurred in 41% of pregnancies (with an additional 21% ongoing beyond 30 weeks). There was a 64% vaginal delivery rate and no low-birth-weight infants. There was no specific pattern of complications and, although two patients had placental problems, both had standard obstetric risk factors for this outcome unlike the uterine artery embolization participants. As a result, the Food and Drug Administration-approved indication for magnetic resonance-guided focused ultrasonography treatment of uterine leiomyomas has been updated to include women who desire future fertility after appropriate counseling.
Conclusions
Leiomyomas are an important disease and are increasingly treated with alternatives to hysterectomy. Understanding the diversity of disease in both pathophysiology and symptomatology will lead to targeted treatment in the short-term and prevention strategies in the long-term. We have made a great deal of progress in the last 20 years but still have far to go. We aim for the time when evidence suggests surgery will be clearly indicated for some women and lifestyle modification is adequate for others.
Acknowledgments
Supported by RC1HD063312 and R01HD060503 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health and NIH/NCRR CTSA Grant No. UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Financial Disclosure: Dr. Stewart has been a Clinical Trial Investigator for Insightec, a consultant to Abbott and Gynesonics, and has served on the scientific advisory board for the Bayer HealthCare Scientific Committee. Dr. Laughlin did not report any potential conflicts of interest.
References
- 1.Speert H. Obstetrics and gynecology in America: a history. Chicago (IL): American College of Obstetricians and Gynecologists; 1980. [Google Scholar]
- 2.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–34. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson GF, Shaber RE, Armstrong MA, Hung YY. Hysterectomy rates for benign indications. Obstet Gynecol. 2006;107:1278–83. doi: 10.1097/01.AOG.0000210640.86628.ff. [DOI] [PubMed] [Google Scholar]
- 4.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–31. [PubMed] [Google Scholar]
- 5.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 6.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskenazi B, Warner M, Samuels S, Young J, Gerthoux PM, Needham L, et al. Serum dioxin concentrations and risk of uterine leiomyoma in the Seveso Women's Health Study. Am J Epidemiol. 2007;166:79–87. doi: 10.1093/aje/kwm048. [DOI] [PubMed] [Google Scholar]
- 8.Huyck KL, Panhuysen CI, Cuenco KT, Zhang J, Goldhammer H, Jones ES, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198(168):e1–9. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas.Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–90. [PubMed] [Google Scholar]
- 10.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–73. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–55. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M, et al. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab. 2009;94:1752–6. doi: 10.1210/jc.2008-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Q, Luo X, Chegini N. Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol. 2007;5:34. doi: 10.1186/1477-7827-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82:736–40. [PubMed] [Google Scholar]
- 15.Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105:19887–92. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aharoni A, Reiter A, Golan D, Paltiely Y, Sharf M. Patterns of growth of uterine leiomyomas during pregnancy.A prospective longitudinal study. Br J Obstet Gynaecol. 1988;95:510–3. doi: 10.1111/j.1471-0528.1988.tb12807.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy.A sonographic study. J Ultrasound Med. 1992;11:511–5. doi: 10.7863/jum.1992.11.10.511. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin SK, Herring AH, Savitz DA, Olshan AF, Fielding JR, Hartmann KE, et al. Pregnancy-related fibroid reduction. Fertil Steril. 2010;94:2421–3. doi: 10.1016/j.fertnstert.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–50. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- 20.Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–23. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–9. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 22.Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414–8. [PubMed] [Google Scholar]
- 23.Alternatives to hysterectomy in the management of leiomyomas. ACOG Practice Bulletin. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;112:387–400. doi: 10.1097/AOG.0b013e318183fbab. [DOI] [PubMed] [Google Scholar]
- 24.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 25.Stewart EA. Uterine fibroids: the complete guide. Baltimore (MD): Johns Hopkins University Press; 2007. [Google Scholar]
- 26.Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med. 2010;28:260–73. doi: 10.1055/s-0030-1251483. [DOI] [PubMed] [Google Scholar]
- 27.Stewart EA, Morton CC. The genetics of uterine leiomyomata: what clinicians need to know. Obstet Gynecol. 2006;107:917–21. doi: 10.1097/01.AOG.0000206161.84965.0b. [DOI] [PubMed] [Google Scholar]
- 28.Rein MS, Powell WL, Walters FC, Weremowicz S, Cantor RM, Barbieri RL, et al. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4:83–6. doi: 10.1093/molehr/4.1.83. [DOI] [PubMed] [Google Scholar]
- 29.Brosens I, Deprest J, Dal Cin P, Van den Berghe H. Clinical significance of cytogenetic abnormalities in uterine myomas. Fertil Steril. 1998;69:232–5. doi: 10.1016/s0015-0282(97)00472-x. [DOI] [PubMed] [Google Scholar]
- 30.Hodge JC, Park PJ, Dreyfuss JM, Assil-Kishawi I, Somasundaram P, Semere LG, et al. Identifying the molecular signature of the interstitial deletion 7q subgroup of uterine leiomyomata using a paired analysis. Genes Chromosomes Cancer. 2009;48:865–85. doi: 10.1002/gcc.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–79. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- 32.Stewart EA, Nowak RA. New concepts in the treatment of uterine leiomyomas. Obstet Gynecol. 1998;92:624–7. doi: 10.1016/s0029-7844(98)00243-9. [DOI] [PubMed] [Google Scholar]
- 33.Stewart EA, Nowak RA. Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Hum Reprod Update. 1996;2:295–306. doi: 10.1093/humupd/2.4.295. [DOI] [PubMed] [Google Scholar]
- 34.Lee BS, Nowak RA. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab. 2001;86:913–20. doi: 10.1210/jcem.86.2.7237. [DOI] [PubMed] [Google Scholar]
- 35.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–20. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol (Oxf) 2008;69:462–70. doi: 10.1111/j.1365-2265.2008.03207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BS, Margolin SB, Nowak RA. Pirfenidone: a novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab. 1998;83:219–23. doi: 10.1210/jcem.83.1.4503. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, Al-Hendy M, Richard-Davis G, Montgomery-Rice V, Sharan C, Rajaratnam V, et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am J Obstet Gynecol. 2010;202(289):e1–9. doi: 10.1016/j.ajog.2009.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blauer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91:1919–25. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- 40.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II.Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153:11–9. doi: 10.1093/aje/153.1.11. [DOI] [PubMed] [Google Scholar]
- 41.Laughlin SK, Schroeder JC, Baird DD. New directions in the epidemiology of uterine fibroids. Semin Reprod Med. 2010;28:204–17. doi: 10.1055/s-0030-1251477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viswanathan M, Hartmann K, McKoy N, Stuart G, Rankins N, Thieda P, et al. Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep) 2007;154:1–122. [PMC free article] [PubMed] [Google Scholar]
- 43.Zapata LB, Whiteman MK, Tepper NK, Jamieson DJ, Marchbanks PA, Curtis KM. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41–55. doi: 10.1016/j.contraception.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Lakhani KP, Marsh MS, Purcell W, Hardiman P. Uterine artery blood flow parameters in women with dysfunctional uterine bleeding and uterine fibroids: the effects of tranexamic acid. Ultrasound Obstet Gynecol. 1998;11:283–5. doi: 10.1046/j.1469-0705.1998.11040283.x. [DOI] [PubMed] [Google Scholar]
- 45.Sundstrom A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. BJOG. 2009;116:91–7. doi: 10.1111/j.1471-0528.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 46.Ip PP, Lam KW, Cheung CL, Yeung MC, Pun TC, Chan QK, et al. Tranexamic acid-associated necrosis and intralesional thrombosis of uterine leiomyomas: a clinicopathologic study of 147 cases emphasizing the importance of drug-induced necrosis and early infarcts in leiomyomas. Am J Surg Pathol. 2007;31:1215–24. doi: 10.1097/PAS.0b013e318032125e. [DOI] [PubMed] [Google Scholar]
- 47.El-Nashar SA, Hopkins MR, Creedon DJ, St Sauver JL, Weaver AL, McGree ME, et al. Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol. 2009;113:97–106. doi: 10.1097/AOG.0b013e31818f5a8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka YO, Nishida M, Tsunoda H, Okamoto Y, Yoshikawa H. Smooth muscle tumors of uncertain malignant potential and leiomyosarcomas of the uterus: MR findings. J Magn Reson Imaging. 2004;20:998–1007. doi: 10.1002/jmri.20207. [DOI] [PubMed] [Google Scholar]
- 49.Bedient CE, Magrina JF, Noble BN, Kho RM. Comparison of robotic and laparoscopic myomectomy. Am J Obstet Gynecol. 2009;201(566):e1–5. doi: 10.1016/j.ajog.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 50.van der Kooij SM, Hehenkamp WJ, Volkers NA, Birnie E, Ankum WM, Reekers JA. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 5-year outcome from the randomized EMMY trial. Am J Obstet Gynecol. 2010;203(105):e1–13. doi: 10.1016/j.ajog.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 51.Edwards RD, Moss JG, Lumsden MA, Wu O, Murray LS, Twaddle S, et al. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360–70. doi: 10.1056/NEJMoa062003. [DOI] [PubMed] [Google Scholar]
- 52.Smeets AJ, Nijenhuis RJ, van Rooij WJ, Weimar EA, Boekkooi PF, Lampmann LE, et al. Uterine artery embolization in patients with a large fibroid burden: long-term clinical and MR follow-up. Cardiovasc Intervent Radiol. 2010;33:943–8. doi: 10.1007/s00270-009-9793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katsumori T, Kasahara T, Tsuchida Y, Nozaki T. Amenorrhea and resumption of menstruation after uterine artery embolization for fibroids. Int J Gynaecol Obstet. 2008;103:217–21. doi: 10.1016/j.ijgo.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–87. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 55.Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, et al. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006;108:930–7. doi: 10.1097/01.AOG.0000234651.41000.58. [DOI] [PubMed] [Google Scholar]
- 56.Lee DW, Ozminkowski RJ, Carls GS, Wang S, Gibson TB, Stewart EA. The direct and indirect cost burden of clinically significant and symptomatic uterine fibroids. J Occup Environ Med. 2007;49:493–506. doi: 10.1097/JOM.0b013e31805f6cf2. [DOI] [PubMed] [Google Scholar]
- 57.Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483–91. doi: 10.1097/00006254-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 58.Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67–76. doi: 10.1097/01.AOG.0000149156.07061.1f. [DOI] [PubMed] [Google Scholar]
- 59.Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22:1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 60.Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199–209. doi: 10.1016/j.fertnstert.2008.10.001. [DOI] [PubMed] [Google Scholar]
Suggested Reading List
- Alternatives to hysterectomy in the management of leiomyomas. ACOG Practice Bulletin. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2008;112:387–400. doi: 10.1097/AOG.0b013e318183fbab. [DOI] [PubMed] [Google Scholar]
- Catherino WH, editor. Insights and advances in uterine leiomyomas. New York (NY): Thieme; 2010. [DOI] [PubMed] [Google Scholar]
- Goodwin SC, Spies JB. Uterine fibroid embolization. N Engl J Med. 2009;361:690–7. doi: 10.1056/NEJMct0806942. [DOI] [PubMed] [Google Scholar]
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. The Cochrane Database of Systematic Reviews. 2000;(4) doi: 10.1002/14651858.CD000249. Art. No.: CD000249. [DOI] [PubMed] [Google Scholar]
- Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci U S A. 2008;105:19887–92. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritts EA. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv. 2001;56:483–91. doi: 10.1097/00006254-200108000-00022. [DOI] [PubMed] [Google Scholar]
- Rabinovici J, David M, Fukunishi H, Morita Y, Gostout BS, Stewart EA. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199–209. doi: 10.1016/j.fertnstert.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids: the complete guide. Baltimore (MD): Johns Hopkins University Press; 2007. [Google Scholar]
- Viswanathan M, Hartmann K, McKoy N, Stuart G, Rankins N, Thieda P, et al. Management of uterine fibroids: an update of the evidence. Evid Rep Technol Assess (Full Rep) 2007;154:1–122. [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]


