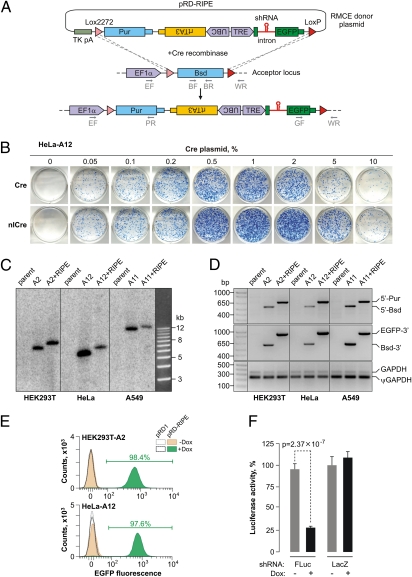
Fig. 2.
Developing the HILO-RMCE technology. (A) Diagram of the HILO-RMCE reaction using the pRD-RIPE donor plasmid. (B) HeLa-A12 cells containing the RMCE acceptor locus were cotransfected in a 12-well format with the pRD-RIPE plasmid and the indicated amounts of the pCAGGS-Cre or pCAGGS-nlCre plasmids. Note that nlCre performed better than the wild-type Cre over a wide concentration range. (C) Genomic DNA was isolated from three parental cell lines (HEK293T, HeLa, and A549; lanes labeled “parent”), the corresponding HILO-RMCE acceptor clones (“A” followed by the clone number), and pooled clones obtained by the HILO-RMCE-mediated integration of the RIPE cassette (the “A+RIPE” lanes). The DNA samples were digested with NcoI and analyzed by Southern blotting to confirm the uniform rearrangement of the acceptor locus as a result of the RMCE reaction. The results are consistent with the expected increase in the length of the acceptor locus-specific NcoI fragment by 856 bp following the RIPE integration. (D) The precision of the RMCE reaction was further confirmed by analyzing the genomic DNA samples described in C by multiplex PCR using either the 5′ (EF, BR, and PR, see A) or the 3′ junction primer mixture (GF, BF, and WR; see A). The primers were designed so that the corresponding PCR product sizes were distinct for the original acceptor (5′-Bsd and Bsd-3′) and the RIPE-targeted loci (5′-Pur and EGFP-3′). GAPDH-specific primers detecting both the bona fide gene (GAPDH) and a pseudogene (ψGAPDH) were used as a control. (E) HILO-RMCE colonies produced by cotransfecting HEK293T-A2 and HeLa-A12 cells with pCAGGS-nlCre and either pRD1 or pRD-RIPE were pooled and incubated with 2 μg/mL Dox for 48 h or left untreated. The cellular EGFP expression was then examined by FACS. Note that nearly all cells express EGFP in the Dox-treated pRD-RIPE samples. (F) HEK293T-A2 cells carrying RIPE cassettes with shRNAs against either FLuc or LacZ were induced with Dox for 36 h or left untreated. The cells were then transfected with a mixture of plasmids encoding the FLuc and RLuc luciferases and the normalized FLuc activities were assayed as described in SI Materials and Methods. Data are averaged from six transfection experiments ± SD.