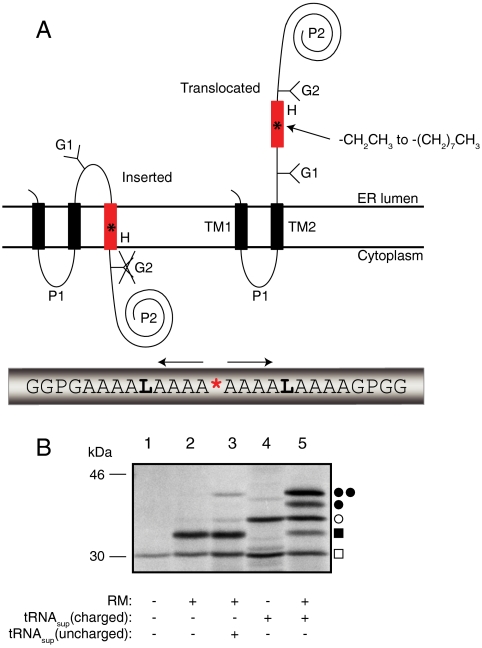
Fig. 1.
(A) Model H segments are introduced into the Lep host protein between two engineered acceptor sites for N-linked glycosylation (G1, G2). A typical H segment is shown, including a nonproteinogenic amino acid (*) and GGPG…GPGG flanking regions. The fractions of membrane-inserted and noninserted H segment are determined by quantitation of radiolabeled singly and doubly glycosylated forms of the protein. (B) In vitro translation of a Lep construct with the H segment AAAALAAAAAAAAALAAAX (X = Me-Trp) in the absence and presence of charged or uncharged tRNAsup and dog pancreas RMs. □, Truncated Lep protein resulting from termination at the UAG stop codon in the H segment; ▪, truncated Lep protein glycosylated on the G1 site; ○, full-length, nonglycosylated Lep protein; ●, full-length Lep protein glycosylated on the G1 site; ●●, full-length Lep protein glycosylated on the G1 and G2 sites. The degree of suppression in the absence of added tRNAsup is negligible (lane 2), and is very low (4%) with added uncharged tRNAsup (lane 3). Suppression is > 50% in the presence of charged tRNAsup (lanes 4 and 5). The amounts of sample loaded in -RM and +RM lanes were adjusted to give roughly equal signals.