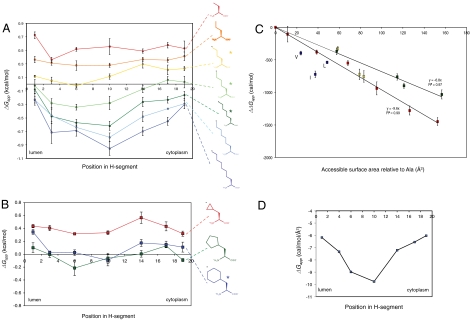
Fig. 2.
(A) Apparent free energy of insertion (ΔGapp) for 19-residue-long H segments carrying a single nonproteinogenic amino acid with a linear alkyl side chain (shown on the right) in the indicated positions. The H segments all have the sequence AAAALAAAAAAAAALAAAA with the Ala in the indicated position replaced by the nonproteinogenic amino acid. The lumenal, N-terminal end of the H segment is on the left and the cytoplasmic end on the right. Error bars show standard deviations; * indicates side chains for which the ΔGapp value for position 6 is significantly smaller than the value for position 14 (two-sided t test, p < 0.01), i.e., that have a significant asymmetry in the ΔGapp profile. (B) ΔGapp for cyclic alkyl side chains. The H segments all have the sequence AAAALAAAAAAAAALAAAA with the Ala in the indicated position replaced by the nonproteinogenic amino acid. (C) ΔGapp decreases in proportion to the increase in ASA of the nonproteinogenic amino acid. ΔΔGapp is measured relative to the AAAALAAAAAAAAALAAAA H segment and the nonproteinogenic amino acid is in the middle position (position 10) in the H segment. The change in ASA is calculated relative to a model AAAALAAAAAAAAALAAAA α-helix. Red data points are for linear alkyl side chains, yellow for cyclic alkyl side chains (cyclopropyl, cyclopentyl, cyclohexyl), green for aromatic side chains lacking polar groups (Phe, 1-naphthyl, 2-naphthyl, biphenyl), and blue for the branched natural amino acids Leu, Ile, and Val. The average change in ΔGapp is -9.6 cal/(mol·Å2) for the linear alkyl side chains and -6.8 cal/(mol·Å2) for the nonpolar aromatic side chains. (D) Average change in ΔGapp per square angstrom (Å2) of accessible surface area as a function of position in the H segment (calculated from the data in A using linear regression as in C).