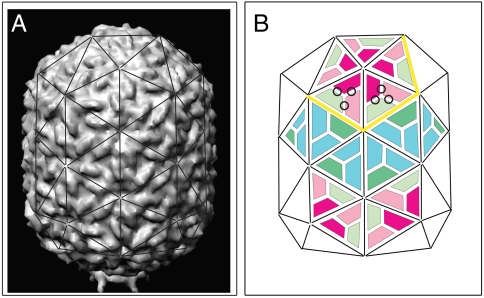
Fig. 1.
Protein organization of Φ29 proheads. (A) Three-dimensional surface representation of a fiberless Φ29 prohead reconstructed from cryoelectron microscopy (22) with superimposed triangulation net, viewed perpendicular to the fivefold axis of the particle (based on European Bioinformatics Institute, EMD-1120, EMD-1117). The prohead is 45-nm wide, 54-nm long, and elongated along one fivefold symmetry axis; wall thickness ca. 1.6 nm (18, 22, 32). (B) Schematic representation of gp8 monomer organization on the triangulation net of the Φ29 prohead: Monomers involved in intrapentameric interactions (magenta), hexamer units interacting with pentamer subunits (pink), hexamer interacting with other hexamer units (green), and equatorial subunits interacting in two additional conformations (dark-green, blue). Some monomer junctions are labeled with black circles, some edges around one pentameric plate by a yellow line.