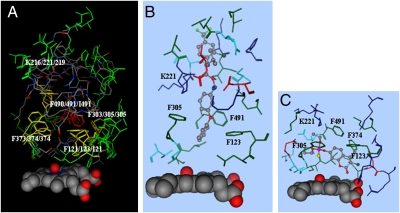
Fig. 2.
Docking of tau-fluvalinate in the CYP9Q2 catalytic site. (A) The docking mode for tau-fluvalinate (elemental colors in stick format) in the predicted CYP9Q2 catalytic site is shown with substrate contacts within 4.5 Å of this substrate. Overlaid with this are the predicted CYP9Q1 and CYP9Q3 catalytic sites with Phe123, Phe305, Phe374, Phe491, and Lys221 conserved in two or three CYP9Q proteins, shown in yellow, and other side chains conserved in all three CYP9Q proteins, shown in green. The heme and its bound oxygen are shown in space-filling format. (B and C) The predicted docking modes for tau-fluvalinate (B) and coumaphos (C) in CYP9Q2 are shown in elemental colors, with amino acid side chains within 4.5 Å of this substrate shown in red for acidic residues, blue for basic residues, green for hydrophobic residues, and cyan for hydrophilic residues.