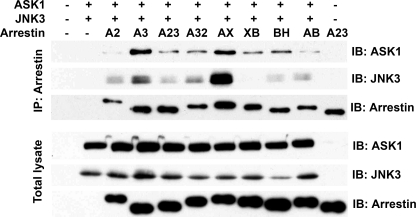
FIGURE 3.
Strength of binding of arrestin to ASK1 and JNK3 does not predict its ability to promote JNK3 phosphorylation. COS-7 cells were transfected with HA-ASK1, HA-JNK3, and the indicated arrestins with a C-terminal FLAG tag or empty vector; harvested 48 h post-transfection; and lysed. Arrestins were immunoprecipitated (IP) with anti-FLAG antibody, and the amount of arrestins and co-immunoprecipitated HA-ASK1 and HA-JNK3 was determined by Western blotting (upper three blots). The expression of these proteins was determined in cell lysates (lower three blots). Cells transfected with empty vector only (first lane), HA-ASK1 + HA-JNK3 only (second lane), or arrestin only (last lane) served as controls. The results of a representative experiment of three performed are shown. Note that the level of JNK3 or ASK1 co-immunoprecipitated with arrestins does not correlate with their ability to promote JNK3 phosphorylation (Figs. 1 and 2): the virtually inactive Arr2-3AX (AX) chimera brought down more ASK1 and JNK3 than fully active arrestin-3, whereas the Arr2N3C (A23) and Arr2-3BH (BH) chimeras, which showed ∼70% and none of arrestin-3 (A3) activity, respectively, co-immunoprecipitated virtually the same amounts of both kinases. A2, arrestin-2; A32, Arr3N2C; XB, Arr2-3XB; AB, Arr2-3AB (detailed chimera descriptions are in the legend to Fig. 1). IB, immunoblot.