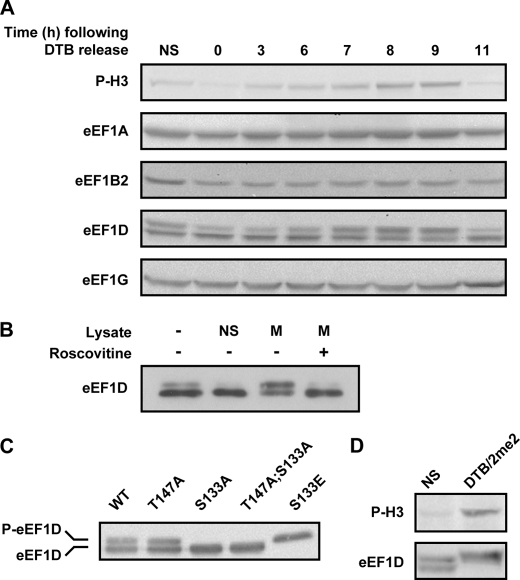
FIGURE 4.
Level and migration pattern of eEF1 subunits. A, HeLa cells were synchronized to the G1/S boundary using DTB and harvested at the indicated time points following release from the block. 40 μg of total protein at each time point were subjected to immunoblot analysis using antibodies specific to the indicated proteins. B, eEF1D was immunoprecipitated from non-synchronized HeLa cells expressing FLAG-tagged wild-type eEF1D. The Sepharose beads-associated FLAG-eEF1D was then incubated with buffer alone or with lysate from non-synchronized (NS) or mitotic (M) HeLa cells either in the absence or presence of roscovitine, a specific CDK1 inhibitor. The beads-associated FLAG-eEF1D was then subjected to immunoblot analysis using anti-FLAG antibody. C, total protein from HeLa cells stably expressing FLAG-tagged eEF1D wild-type and T147A, S133A, T147A;S133A, and S133E was subjected to immunoblot analysis using anti-FLAG antibody. D, total protein from non-synchronized (NS) and mitotic (DTB/2me2) HeLa cells was subjected to immunoblot analysis using anti-phospho-H3 (P-H3) and anti-eEF1D antibodies.