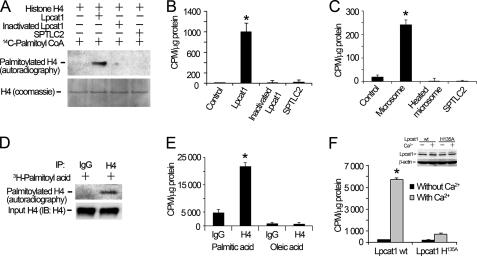
FIGURE 2.
Lpcat1 catalyzes histone H4 protein palmitoylation in vitro and in vivo. A, in vitro palmitoylation. Histone H4 protein palmitoylation reactions were conducted in the presence of Lpcat1, heat-inactivated (denatured) Lpcat1, and a related palmitoyltransferase, SPTLC2, using [14C]palmitoyl-CoA as a donor and recombinant histone H4 substrate. The lower panel shows histone H4 protein input controls. B, the relevant bands on nitrocellulose membranes were cut, and the radioactivity was counted using a scintillation counter. *, p = 0.0017, radioactivity of Lpcat1 versus dpm of heat-inactivated Lpcat1. C, histone H4 protein palmitoylation reactions were conducted in the presence of lung microsomes, heat-inactivated Lpcat1, and SPTLC2. *, p = 0.0002, radioactivity of microsome versus dpm of heat-inactivated microsome. D, in vivo palmitoylation. MLE cells were pulse-labeled with [3H]palmitoyl acid in the presence of 2 mm Ca2+ for 2 h. Cell lysates were immunoprecipitated (IP) with anti-H4 antibody or IgG to detect palmitoylated H4 followed by autoradiography. Cell lysates were analyzed by V5 immunoblotting as an input control in the lower panel. E, MLE cells were pulse-labeled with [3H]palmitoyl acid or [3H]oleic acid in the presence or absence of 2 mm Ca2+ for 2 h. Cell lysates were immunoprecipitated with H4 antibody, and the radioactivity of the precipitates was measured by scintillation counting. *, p = 0.017, radioactivity of H4 in palmitic acid group versus oleic acid group. F, HEK 293 cells were transfected with pcDNA3.1/Lpcat1 or a Lpcat1 catalytically inactive mutant (Lpcat1 H135A) for 24 h. Cells were pulse-labeled with [3H]palmitoyl acid with or without Ca2+ as above. Histone radioactivity was determined as described in E. The inset shows the protein expression levels of Lpcat1 wt and Lpcat1 H135A mutant and β-actin. *, p = 0.0005, radioactivity of wild type Lpcat1 versus dpm of H135A Lpcat1. The data represent three independent experiments. Error bars, S.E.