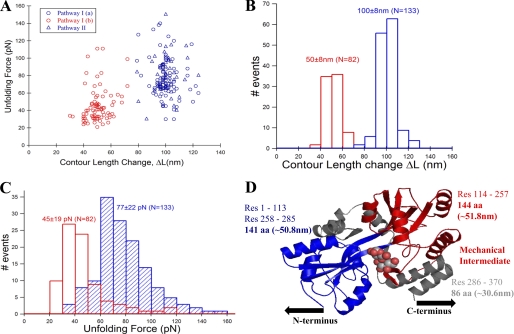
FIGURE 3.
The intermediate on path I of MBP unfolding is mechanically weaker than the native protein. A, unfolding force and contour length change values of MBP obtained from many single-molecule FX traces (see Fig. 2, A and B) were used in making unfolding force versus contour length change scatter plot. The plot clearly shows two clusters (or populations) of force peaks: one for the initial unfolding event (blue circles from path I and blue triangles from path II) and another for the intermediate unfolding event (red circles, only from path I). B, contour length change histograms for the initial (ΔLM = 100 ± 8 nm, blue bars) and intermediate states (ΔLI = 50 ± 8 nm, red bars). C, unfolding force histograms of the initial (77 ± 22 pN, blue bars) and intermediate unfolding (45 ± 19 pN, red bars). Unfolding force of initial peak from both path I (blue circles) and II (blue triangles) are combined, indicating that they are mechanically indistinct. The intermediate is mechanically weaker than the native state of MBP. D, proposed contour length mapping onto MBP structure (maltose-bound MBP structure Protein Data Bank ID code 1ANF). Protein region labeled gray likely gives the ∼28-nm low unfolding force peaks preceding the initial force peak. Path I: N-terminal domain (colored blue) manifests in the initial peak (N→I), and C-terminal domain (colored red) gives the intermediate unfolding (I→U). However, in path II, both N- and C-terminal domains unfold together giving a single peak (N→U) without any on-pathway intermediate.