FIGURE 3.
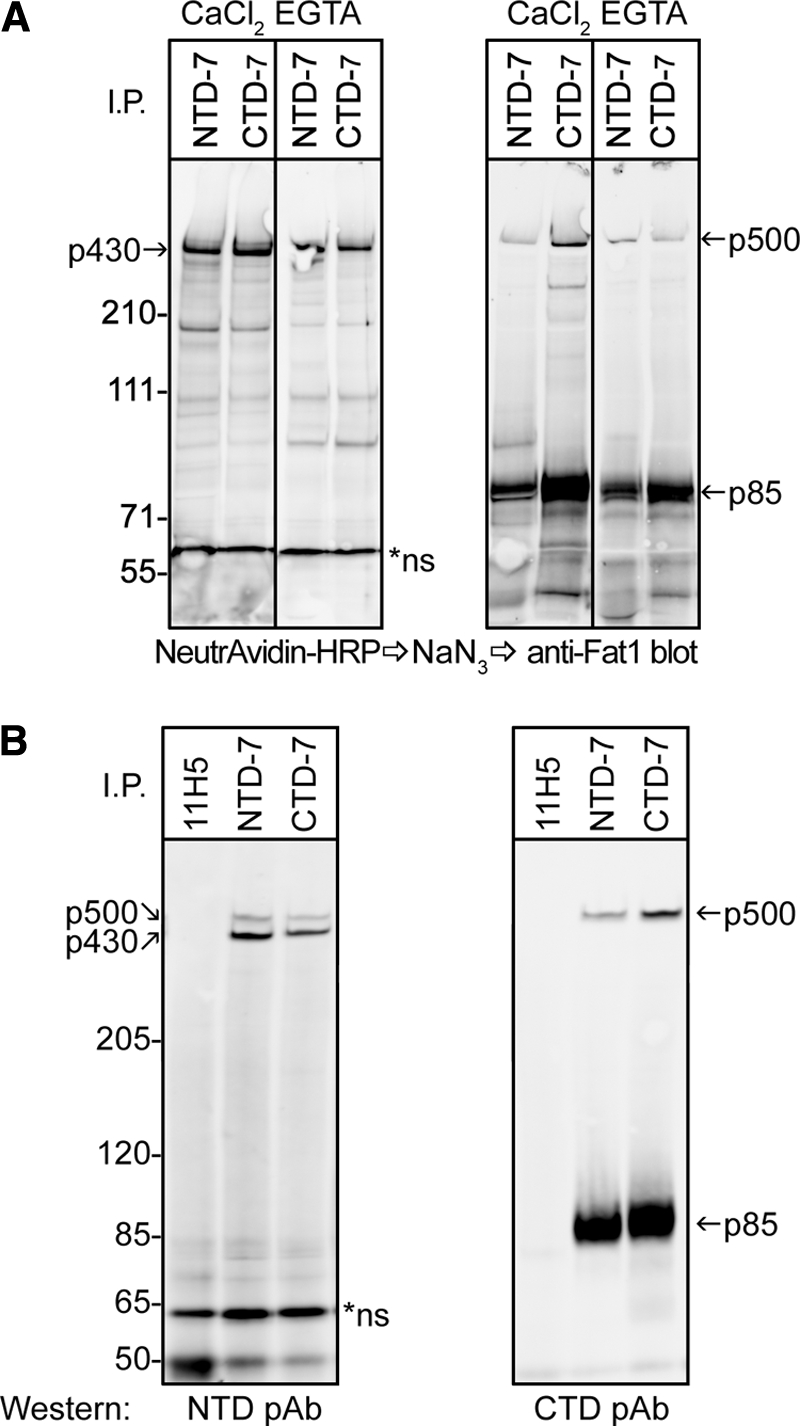
FAT1 cadherin is cleaved and expressed as a heterodimer on cell surface of keratinocytes. A, cell surface proteins of HaCaT keratinocytes were labeled in situ with biotin before being subjected to lysis and immunoprecipitation (I.P.) using mAbs directed against epitopes in the extracellular domain and cytoplasmic tail of FAT1, NTD-7 and CTD-7, respectively. CaCl2 and EGTA indicate the presence (2 mm CaCl2) or absence (5 mm EGTA) of calcium ions in the immunoprecipitation buffers used. Membranes were first probed with NeutrAvidin-HRP to reveal cell surface proteins (left panels) followed by quenching HRP activity with azide and subsequently reprobing membranes with FAT1 C-terminal pAbs by Western blot (right panels). Both mAbs precipitated a band at ∼430 kDa (p430) on the cell surface, whereas reblotting using the C-terminal pAbs showed a ∼500-kDa reactive band (p500) and a strong ∼85-kDa band (p85). B, cell lysates of HaCaT cells immunoprecipitated with NTD-7, CTD-7, and control mAbs (11H5) were subjected to Western blotting with either FAT1 N-terminal pAbs or C-terminal pAbs. The N-terminal pAbs recognize both p430 and p500 bands in both NTD-7 and CTD-7 immunoprecipitates in contrast to the C-terminal pAbs that recognize only p500 and p85.
