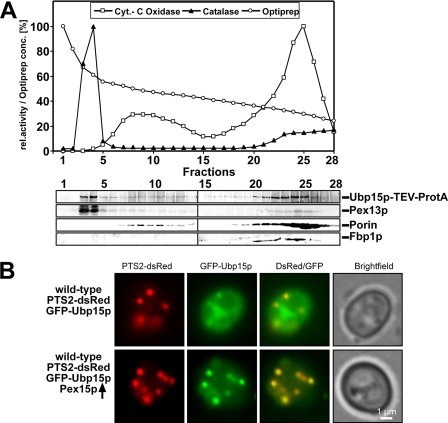
FIGURE 3.
Ubp15p is partially localized to peroxisomes. A, a cell-free extract of oleate-induced wild-type cells expressing genomically tagged Ubp15p (Ubp15p-TEV-ProtA) was separated by density gradient centrifugation (2.25–24% OptiPrep, 18% sucrose). Fractions were subjected to measurements of the activity of catalase and cytochrome (Cyt.) c oxidase as peroxisomal and mitochondrial markers, respectively (upper panel). Equal portions of fractions were probed by immunoblotting (lower panel) with antibodies against the protein A tag, Pex13p (peroxisomes), porin (mitochondria), and Fbp1p (cytosol). B, wild-type cells expressing both the PTS2 marker protein PTS2-dsRed and GFP-Ubp15p with and without overexpression of Pex15p were grown on oleic acid plates for 2 days and examined by fluorescence microscopy. Although only a small portion of GFP-Ubp15p was localized to peroxisomes in cells containing normal levels of Pex15p, a higher fraction of the fusion protein was recruited to peroxisomes upon overexpression of Pex15p as indicated by the co-localization of GFP-Ubp15p and the peroxisomal dsRed marker. rel., relative.