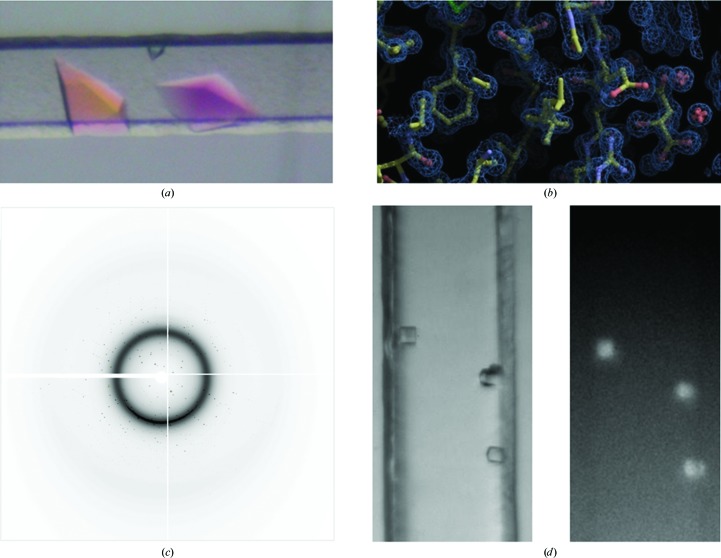
Figure 3.
(a) Crystals of thaumatin were grown from 0.8 M potassium sodium tartrate, 0.1 M HEPES pH 7.5 in the Crystal Former. Here they are shown under polarized light. (b) Representative 2F o − F c electron density for the refined thaumatin structure at 1.25 Å resolution shown with 1.5σ contours. (c) The diffraction pattern of lysozyme crystals grown in the Crystal Former. X-ray data were collected in situ at room temperature on the X6A beamline (National Synchrotron Light Source, Brookhaven National Laboratory, Upton, New York, USA). (d) Detection of protein crystals in the Crystal Former using a UVEX microscope. Brightfield (left) and UV-fluorescence (right) images of lysozyme crystals within the microchannels of the Crystal Formers are shown. The microchannel width is 150 µm and the exposure lengths were 0.5 and 1 s for the brightfield and fluorescence images, respectively.