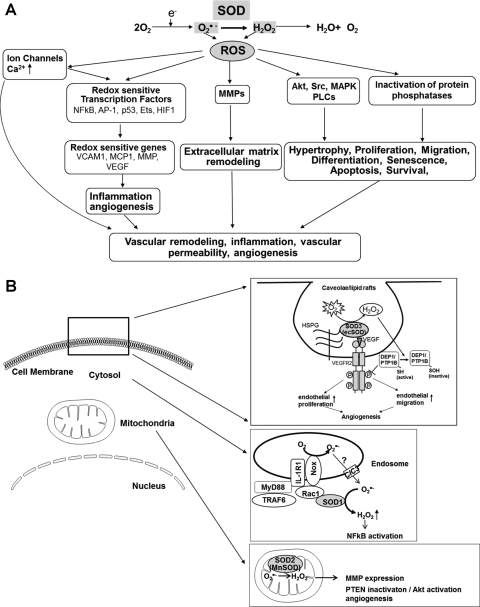
FIG. 7.
(A) Role of SOD in redox-sensitive signaling pathways and (B) role of SODs in activation of redox signaling at specific compartments. (A) Production of O2•− and its metabolite H2O2 lead to activation of redox-sensitive kinases and potentially inactivation of specific phosphatases to modulate redox-sensitive signaling, including hypertrophy, proliferation, and migration. Activation of redox-sensitive transcription factors leads to redox-sensitive changes in expression of proinflammatory genes, such as vascular cellular adhesion molecule 1 (VCAM1), monocyte chemotactic protein 1 (MCP1), and intercellular adhesion molecule 1 (ICAM1). ROS modulate ion channels and, therefore, influence intracellular Ca2+ and K1+ concentrations. Finally, ROS can activate matrix metalloproteinases (MMPs). (B) Extracellular H2O2 generated by SOD3 (ecSOD) localized at caveolae/lipid rafts via binding to heparan sulfate proteoglycans (HSPGs) promotes VEGF receptor type2 (VEGFR2) signaling linked to angiogenesis via oxidative inactivation of protein tyrosine phosphatases (PTPs; DEP1 and PTP1B) (180); SOD1 is recruited to redox active endosomal surface where it binds to Rac1 to regulate Nox2 activity. Thus, SOD1-Nox2-mediated increase in O2•− exits endosomes through chloride channels (ClC3) and SOD1-mediated dismutation of O2•− at the endosomal surface produces the localized H2O2, thereby promoting redox activation of NF-kB (162); MnSOD (SOD2) localizes in mitochondria matrix. SOD2 (MnSOD) overexpression-induced H2O2 induces the tumor suppressor PTEN oxidation, leading to enhanced formation of phosphatidylinositol 3,4,5-triphoshate, resulting in activation of Akt and angiogenesis in vivo (40). Thus, O2•− dismutation to H2O2 by the three isoforms of SODs contributes to activation of specific redox signaling events at distinct compartments.