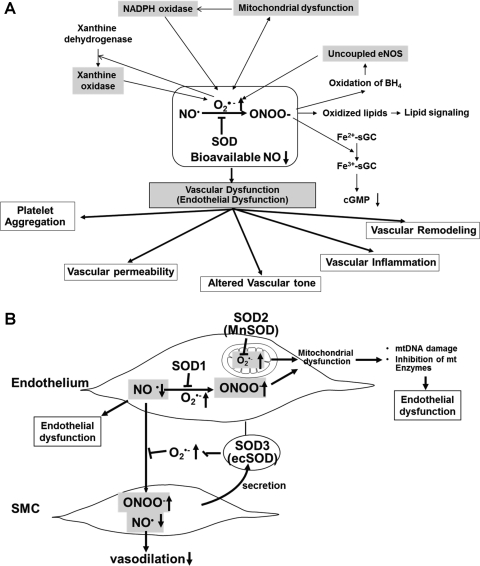
FIG. 8.
(A) Role of nitric oxide–superoxide interactions in vascular (endothelial) dysfunction in cardiovascular disease and (B) protective role of SODs in oxidative stress-dependent vascular (endothelial) dysfunction. (A) NO rapidly reacts with O2•− generated by ROS-generating enzymes, including NADPH oxidase, xanthine oxidase, and mitochondria, to form peroxynitrite anion (ONOO−), which in turn oxidizes various molecules, such as the heme of sGC, lipids, and the endothelial NOS (eNOS) cofactor BH4. This in turn induces uncoupled eNOS to promote further increase in O2•−. These consequences will be further enhanced by interaction of ROS-generating enzymes. Both O2•− and ONOO− promote mitochondrial dysfunction, thereby increasing mitochondrial ROS production. Mitochondria-derived ROS, which in turn further activates NADPH oxidase, results in increased ROS production and reduced NO bioavailability. Further, either O2•− or ONOO− can stimulate other ROS-generating enzymes, such as xanthine oxidase. The loss of bioavailable NO and formation of ONOO− can lead to vascular inflammation, vascular remodeling, altered vascular tone, enhanced vascular permeability, and increased platelet aggregation. These responses are inhibited by SODs. (B) Because of its location, SOD3 (ecSOD) plays a critical role in preventing O2•−-mediated destruction of NO• released from the endothelium at the extracellular space, whereas SOD1 preserves NO levels within the endothelium. Thus, SODs regulate endothelial function and NO mediating signaling by inhibiting O2•−-mediated inactivation of NO•, thereby increasing bioavailable NO•. Because O2•− and NO• are both radicals and contain unpaired electrons in their outer orbitals, they undergo an extremely rapid, diffusion-limited radical–radical reaction (6.7×109 M−1 s−1, three times faster than the dismutation of O2•− by SOD). This reaction leads to the formation of nitrite, nitrate, and, very importantly, the peroxynitrite anion (ONOO−), which in turn induces endothelial dysfunction, vascular inflammation, vascular remodeling, altered vascular tone, enhanced vascular permeability, and increased platelet aggregation. Both O2•− and ONOO− promote mitochondrial dysfunction, thereby increasing mitochondrial ROS production, reducing NO bioavailability, mitochondrial (mt)DNA damage, and inhibition of mitochondrial enzymes. These events result in endothelial dysfunction.