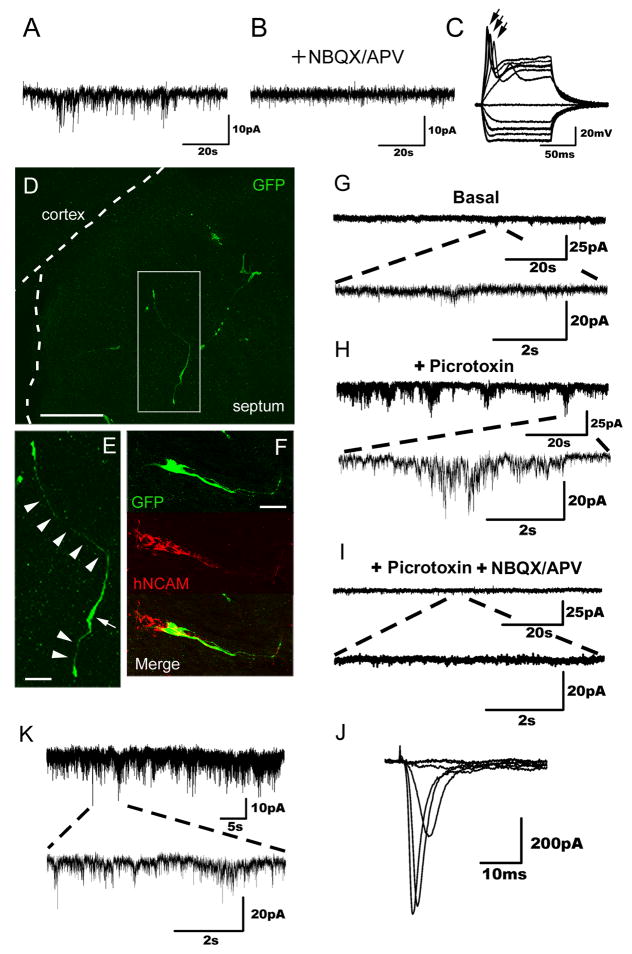
Figure 5. Evidence of hiN cell functional integration.
(A) Representative spontaneous postsynaptic currents recorded from an hiN cell present in a murine glial monolayer co-culture. The cell was held at −70mV. Events of various amplitudes (5–20 pA) are seen.
(B) Spontaneous postsynaptic currents as observed in (A) were abolished by bath application of NBQX/APV.
(C) Upon depolarizing current injections in current-clamp mode, action potentials were induced. Individual traces represent independent recorded events; action potentials (indicated by arrows) were seen in 5 of the 9 tracings.
(D–E) Confocal fluorescent images of brain slices prepared from postnatal day 3 animals that had been grafted in utero with hiN cells. Transplanted hiN cells migrated extensively and extended neurite processes. An arrowhead indicates cell soma; arrows point to apparent processes. Scale bar, 100 μm (D), 20 μm (E).
(F) Confocal reconstruction of a transplanted GFP-positive hiN cell stained with a human-specific NCAM antibody. GFP, green; hNCAM, red; Scale bar, 50 μm.
(G) Voltage clamp recording of an hiN cell (Vh=−70 mV) integrated into the piriform cortex of the host brain, demonstrating spontaneous events of low frequency and amplitude.
(H) The frequency and amplitude of the spontaneous excitatory postsynaptic currents (sEPSCs, as in G) increased upon blockade of GABAA receptors with 50 μM picrotoxin.
(I) sEPSCs were drastically reduced by blocking glutamatergic synaptic transmission with 20 μM NBQX and 50 μM APV.
(J) Sodium currents of the same cell (G–I) elicited by voltage steps from Vh=−70 (−60 to 20 in 10 mV steps).
(K) Representative voltage clamp recording at a holding potential (Vh= −70 mV) of an hiN cell integrated into the cingulate gyrus of the host brain. Traces show spontaneous slow and fast currents of different amplitudes, indicating that this neuron receives synaptic contacts from host cells.