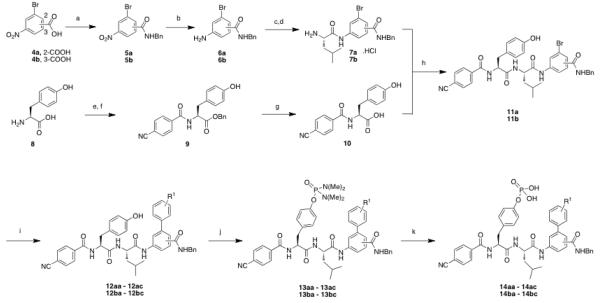
Scheme 1.
(a) benzylamine, HBTU, DIPEA, DMF, 25 °C, 4 hr, 74 % (b) SnCl2, EtOAc, 70 °C, 2 hr, 95 %; (c) (i) N-Boc-Leu-OH, isobutyl chloroformate, CH2Cl2, N-methyl morpholine, 25 °C, 10 min; (ii) (6a, 6b) N-methyl morpholine, CH2Cl2:THF (1:1), 25 °C, 1.5 hr, 96 %; (d) 4M HCl, dioxane:MeOH (1:1), 25 °C, 1 hr, 99 %; (e) benzyl alcohol, p-TsOH.H2O, 110 °C, 24 hr; 95 %; (f) (i) p-cyanobenzoic acid, isobutyl chloroformate, N-methyl morpholine, 25 °C, 15 min; (ii) 8, N-methyl morpholine, CH2Cl2:THF (1:1), 25 °C, 30 min, 84 %; (g) H2, Pd/C, THF:MeOH (1:1) 25 °C, 1 hr, 95 %; (h) HBTU, DIPEA, DMF, 25 °C, 4 hr, 76 %; (i) ArB(OH)2, Pd(PPh3)4, K2CO3, DMF, 170 °C, 15 mins, 50 %; (j) bis(dimethylamino)phosphoramidic chloride, DMAP, DBU, THF: CH2Cl2 (1:1) 25 °C, 16 hr, 62 %; (k) TFA:H2O (9:1), 25 °C, 16 hr, 99 %.