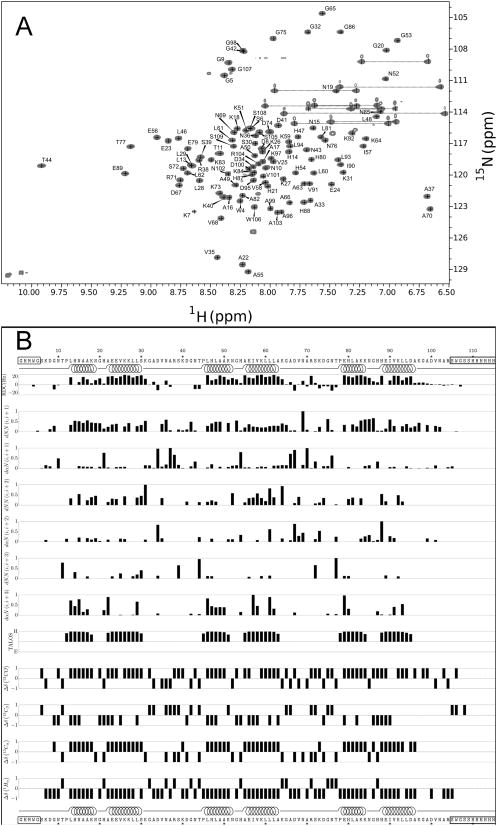
Figure 2. NMR data for a three-repeat consensus ankyrin protein.
(A) 15N-1H HSQC spectrum of uniformly 15N-labelled NRC at 800 MHz (pH 6.5, 25°C). For examples of strip plots used for assignment, see Figure S2. (B) Amide 1H-15N residual dipolar couplings, short- and medium-range NOEs (normalized to the maximum NOE value of each type), predicted secondary structure from TALOS+ (Shen et al., 2009), and chemical shift deviation from random coil-values, calculated using CSI v2.0 (Wishart and Sykes, 1994). Boxed regions of sequence correspond to unstructured regions (horizontal line, Figure 3C). Residues that are helical in the NMR structure, as determined using STRIDE (Heinig and Frishman, 2004), are indicated by spirals.