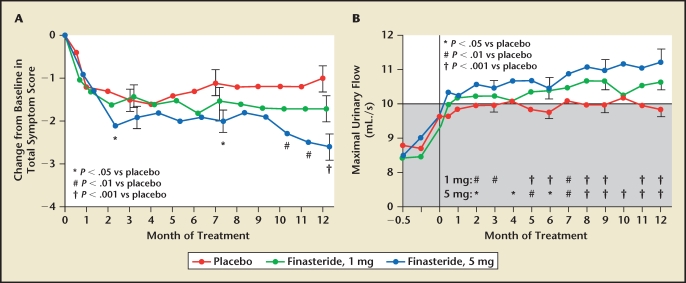
Figure 1.
Trial results comparing placebo and two dosing regimens of finasteride in men with benign prostatic hyperplasia. (A) Men who received finasteride, 5 mg, had a significant decrease in symptom scores at months 2, 7, 10, 11, and 12, compared with placebo. Men who received finasteride, 1 mg, had no significant change in symptom scores. (B) At 6 and 12 months, the maximal flow rates in both finasteride-treated groups were significantly higher than baseline values and rates of the placebo group. Shaded area indicates range in which urinary flow was considered to be obstructed. Reproduced with permission from Gormley GJ et al, N Engl J Med. 1992;327:1185–1191. © Massachusetts Medical Society.