Abstract
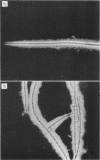
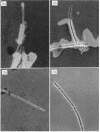
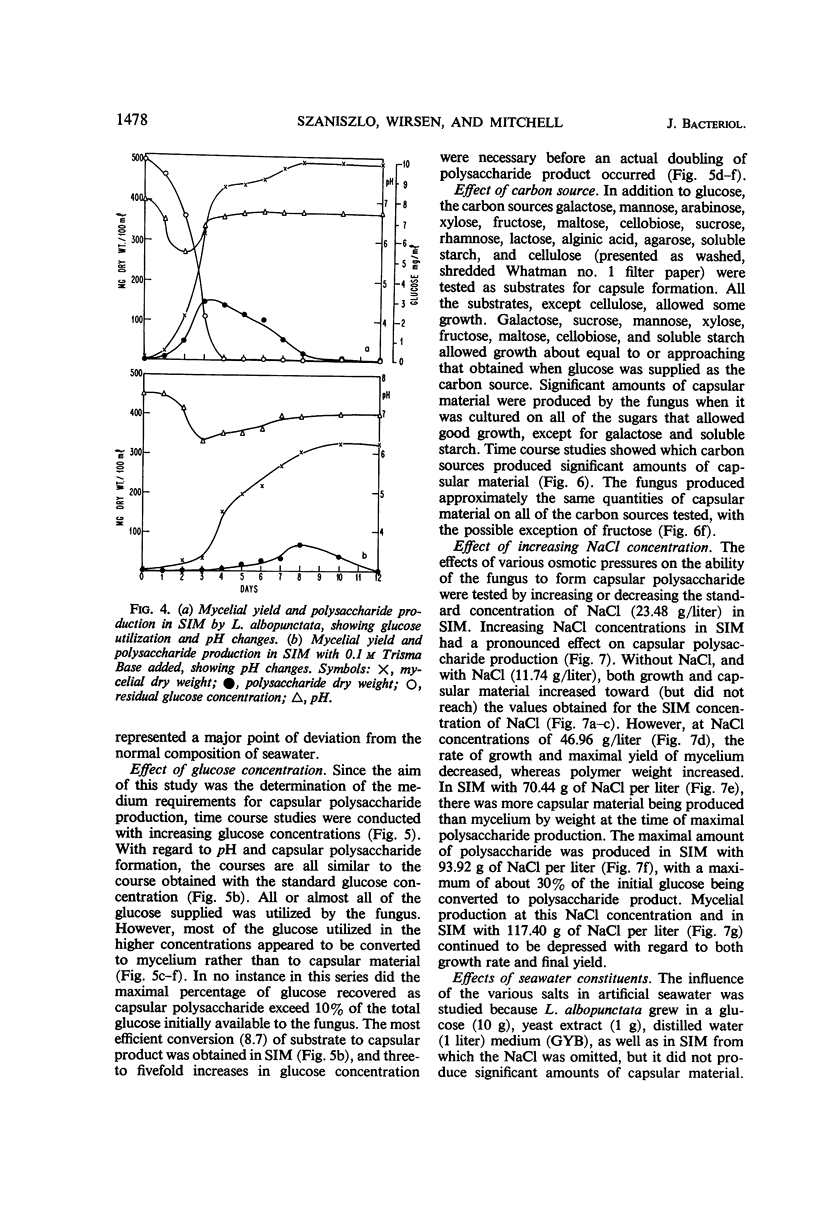
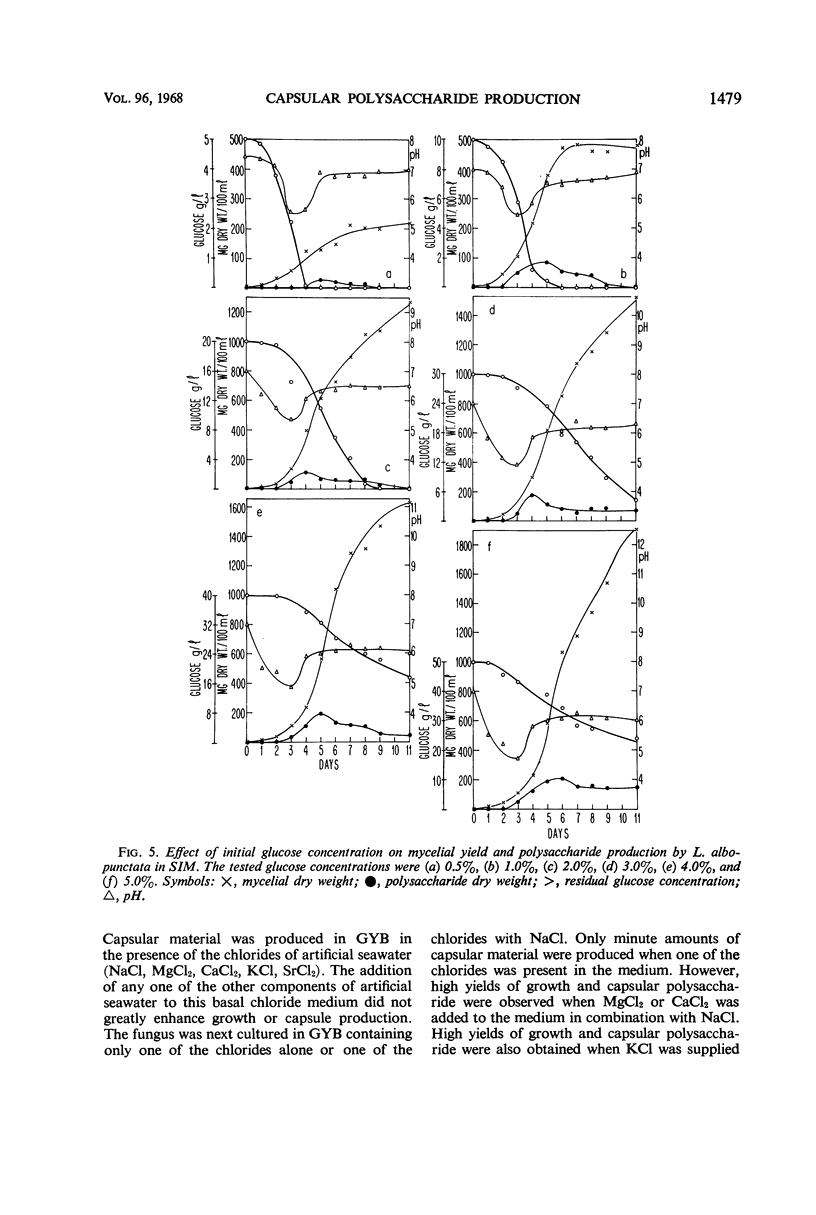
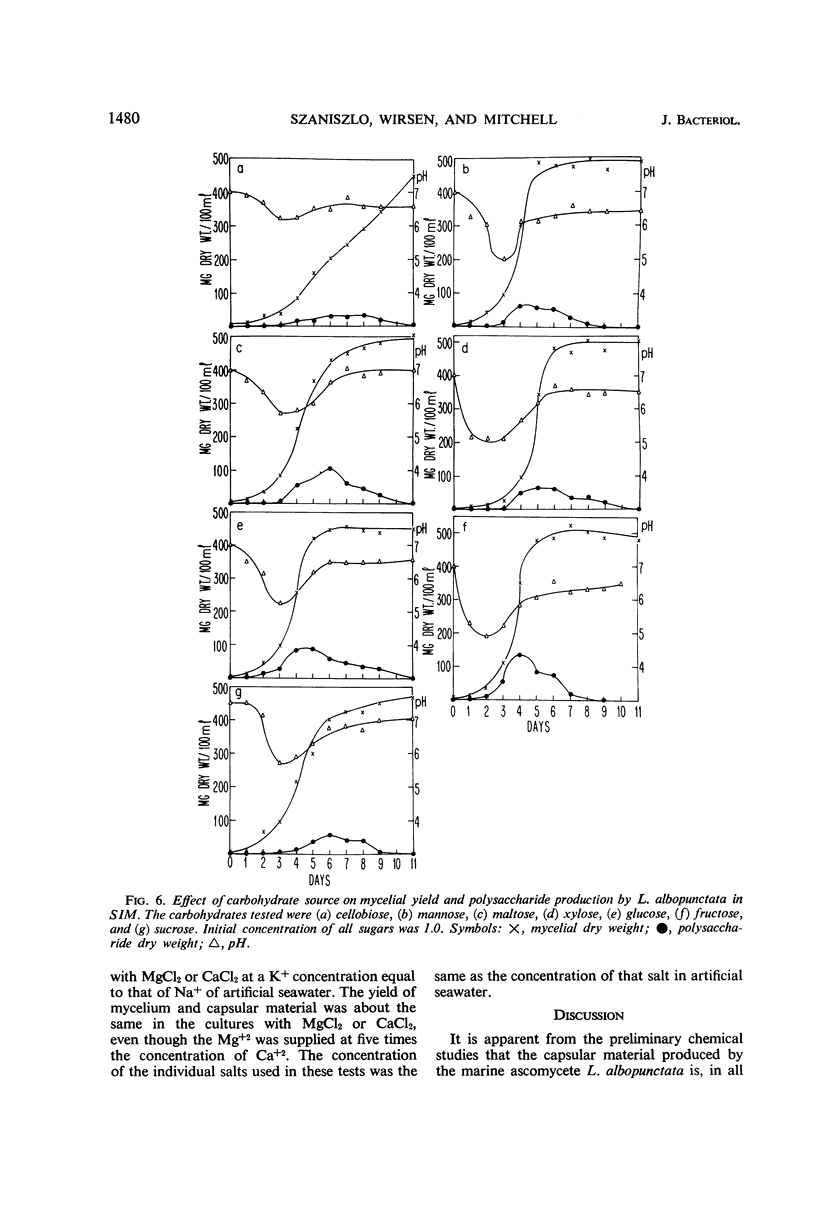
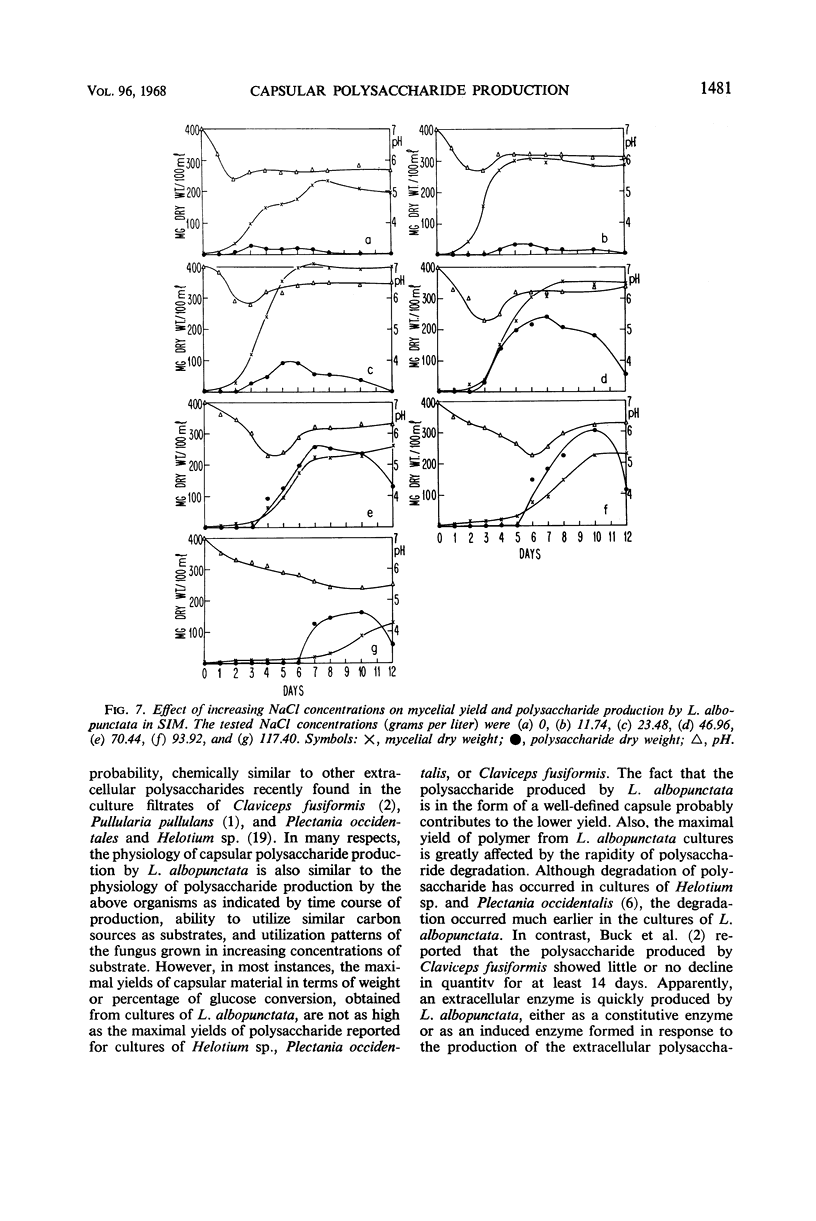
The spent seawater medium of 4-day-old-cultures of the filamentous marine fungus Leptosphaeria albopunctata had a high viscosity after the fungus was collected by high-speed centrifugation. Microscopic examination of uncentrifuged mycelium suspended in India ink revealed that the viscosity resulted from capsular material. These capsules became disassociated from the mycelium during centrifugation. Precipitation of the medium of centrifuged cultures with 95% ethyl alcohol yielded a highly anthrone-positive polysaccharide material, composed of large amounts of glucose and minute amounts of mannose. Time course studies of the nutritional requirements for capsular polysaccharide production revealed that the capsular material was produced in large amounts, and on a wide variety of sugars, during the period of rapid growth, but was quickly degraded and presumably remetabolized in older cultures. The amount of capsular material produced was enhanced by NaCl concentrations above that of artificial seawater, and KCl could be substituted for NaCl. The salts MgCl2 and CaCl2 were also required for capsule production by L. albopunctata, although growth was obtained in cultures without added amounts of these constituents. The possible role of these salts in the metabolism of the fungus is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck K. W., Chen A. W., Dickerson A. G., Chain E. B. Formation and structure of extracellular glucans produced by Claviceps species. J Gen Microbiol. 1968 May;51(3):337–352. doi: 10.1099/00221287-51-3-337. [DOI] [PubMed] [Google Scholar]
- DAVIS E. N., RHODES R. A., SHULKE H. R. FERMENTATIVE PRODUCTION OF EXOCELLULAR GLUCANS BY FLESHY FUNGI. Appl Microbiol. 1965 Mar;13:267–271. doi: 10.1128/am.13.2.267-271.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD R. A., CLARIDGE C. A., HORI A., MURRAY J. F. Observations on the function of sodium in the metabolism of a marine bacterium. J Biol Chem. 1958 Jun;232(2):829–834. [PubMed] [Google Scholar]
- PAYNE W. J. Effects of sodium and potassium ions on growth and substrate penetration of a marine pseudomonad. J Bacteriol. 1960 Nov;80:696–700. doi: 10.1128/jb.80.5.696-700.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. E., Payne W. J. Influence of Na+ on synthesis of a substrate entry mechanism in a marine bacterium. Proc Soc Exp Biol Med. 1967 Mar;124(3):953–955. doi: 10.3181/00379727-124-31894. [DOI] [PubMed] [Google Scholar]
- Sharon N. Polysaccharides. Annu Rev Biochem. 1966;35:485–520. doi: 10.1146/annurev.bi.35.070166.002413. [DOI] [PubMed] [Google Scholar]
- Szaniszlo P. J., Gooder H. Cell wall composition in relation to the taxonomy of some Actinoplanaceae. J Bacteriol. 1967 Dec;94(6):2037–2047. doi: 10.1128/jb.94.6.2037-2047.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON N., MACLEOD R. A. Nutrition and metabolism of marine bacteria. IV. The participation of Na+, K+, and Mg++ salts in the oxidation of exogenous substrates by a marine bacterium. Can J Microbiol. 1957 Jun;3(4):627–638. doi: 10.1139/m57-068. [DOI] [PubMed] [Google Scholar]
- WALLEN L. L., RHODES R. A., SHULKE H. R. PHYSICAL PROPERTIES AND CHEMICAL COMPOSITION OF BETA-GLUCANS FROM FLESHY FUNGI. Appl Microbiol. 1965 Mar;13:272–278. doi: 10.1128/am.13.2.272-278.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]