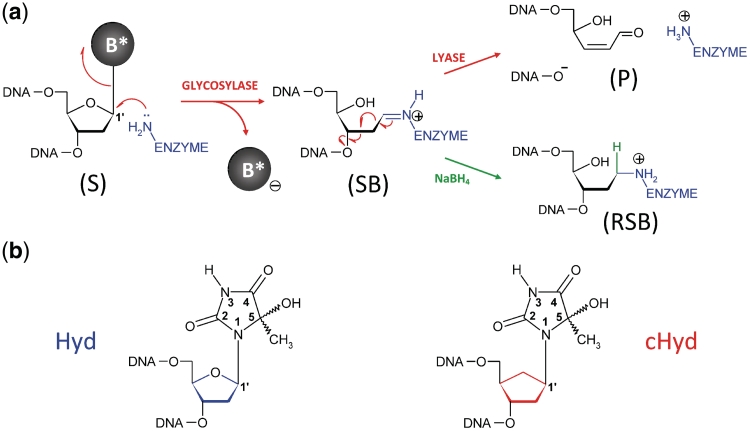
Figure 1.
Repair of oxidized base by DNA glycosylase/AP lyases and structures of the nucleoside used in this study. (a) Glycosylase/lyase process. In the first reaction step, the base lesion (B*) contained in DNA (S, for substrate) was removed by the catalytic cleavage of the N-glycosydic bond (DNA glycosylase). An active site amino group (from an internal lysine or from the N-terminal amino group of the enzyme) was used as a nucleophile. This led to the formation of a covalent imino-enzyme DNA intermediate (SB, for Schiff base) between the C1′ of the damaged nucleoside and the catalytic amino group of the enzyme. In the second step of the reaction, the transient Schiff base intermediate underwent base-catalyzed β-elimination (AP lyase), resulting in strand scission at the C3′ side of the abasic site (P, for DNA cleavage product). In the case of Fpg, the β-elimination product underwent a δ-elimination process leading to the excision of the sugar and the formation of a one-nucleoside gap in DNA (Supplementary Figures S1 and S2). The formation of the Schiff base intermediate can be easily demonstrated by its irreversible stabilization in the presence of sodium borohydride (RSB, for reduced Schiff base). (b) Nucleoside and analogue used in the study. Preparation of 5-Hydroxy-5-methylhydantoin (Hyd, blue) and its carbanucleoside (cHyd, red) and their incorporation in synthetic oligonucleotides are described in the Supplementary Data.