Abstract
The muscarinic receptor subtype M3 is coupled to Gq/11 proteins. Muscarinic receptor agonists such as carbachol stimulate these receptors that result in activation of phospholipase C (PLC) which hydrolyzes phosphatidylinositol 4,5-bisphosphate into diacylglycerol and Ins(1,4,5)P3. This pathway leads to excitation and smooth muscle contraction. In this study the PLC agonist, 2, 4, 6-trimethyl-N-(meta-3-trifluoromethyl-phenyl)-benezenesulfonamide (m-3M3FBS), was used to investigate whether direct PLC activation mimics carbachol-induced excitation.
We examined the effects of m-3M3FBS and 2, 4, 6-trimethyl-N-(ortho-3-trifluoromethyl-phenyl)-benzenesulfonamide (o-3M3FBS), on murine colonic smooth muscle tissue and cells by performing conventional microelectrode recordings, isometric force measurements and patch clamp experiments.
Application of m-3M3FBS decreased spontaneous contractility in murine colonic smooth muscle without affecting the resting membrane potential. Patch clamp studies revealed that delayed rectifier K+ channels were reversibly inhibited by m-3M3FBS and o-3M3FBS. The PLC inhibitor, 1-(6-((17b-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U73122), did not prevent this inhibition by m-3M3FBS. Both m-3M3FBS and o-3M3FBS decreased two components of delayed rectifier K+ currents in the presence of tetraethylammonium chloride or 4-aminopyridine. Ca2+ currents were significantly suppressed by m-3M3FBS and o-3M3FBS with a simultaneous increase in intracellular Ca2+. Pretreatment with U73122 did not prevent the decrease in Ca2+ currents upon m-3M3FBS application.
In conclusion, both m-3M3FBS and o-3M3FBS inhibit inward and outward currents via mechanisms independent of PLC acting in an antagonistic manner. In contrast, both compounds also caused an increase in [Ca2+]i in an agonistic manner. Therefore caution must be employed when interpreting their effects at the tissue and cellular level.
Keywords: smooth muscle, contractility, membrane potential, delayed rectifier K+ channels, L-type Ca2+ current
1. Introduction
Phosphoinositide specific phospholipase C (PLC) plays a critical role in multiple signal transduction pathways resulting in various cellular responses (Rebecchi and Pentyala, 2000; Rhee, 2001; Hicks et al., 2008; Suh et al., 2008). Attempts have been made to identify molecules that can directly modulate PLC activity to elucidate PLC-mediated signaling pathways and important physiological reactions. The compound 2, 4, 6-trimethyl-N-(meta-3-trifluoromethyl-phenyl)-benezenesulfonamide (m-3M3FBS) stimulated generations of superoxide in human neutrophils and this stimulation was through directly activating PLC. 2, 4, 6-trimethyl-N-(ortho-3-trifluoromethyl-phenyl)-benzenesulfonamide (o-3M3FBS), which differs from m-3M3FBS by the position of the trifluoromethyl-phenyl group, does not affect superoxide generation up to 50μM (Bae et al., 2003). m-3M3FBS caused a transient increase in intracellular Ca2+ ([Ca2+]i) in various cell lines including neutrophils, leukocytes and fibroblasts, an effect that was inhibited by the PLC inhibitor, 1-(6-((17b-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U73122). m-3M3FBS also stimulated the formation of inositol phosphates (Bae et al., 2003). In addition, it activated three subfamilies of PLC (β, γ and δ) isoforms in vitro and thus was concluded to show no isoform-specificity. o-3M3FBS did not affect any of these parameters (Bae et al., 2003). However the specificity of m-3M3FBS was questionable when changes in Ca2+ homeostasis by m-3M3FBS were independent of PLC activation (Krjukova et al., 2004). Unlike in the previous report, m-3M3FBS caused a slowly developing Ca2+ elevation which reached a plateau within 6 minutes. The measured increase in PLC activity did not coincide with the elevation in [Ca2+]i since it took more than 20 minutes for generation of inositol 1,4,5-trisphosphate (IP3) (Krjukova et al., 2004). This report concluded that m-3M3FBS was not a selective PLC activator in these cells and its effects on Ca2+ homeostasis were via PLC-independent mechanism(s).
m-3M3FBS has been used to study the role of PLC in the regulation of certain ionic currents. KCNQ2 and 3 currents recorded in transfected tsA cells were suppressed by application of m-3M3FBS (50μM) through activation of PLC whereas o-3M3FBS had no effect on these currents. However, this compound was slow to act, had a long latency and also had a varied response that was suggested to be due to multiple actions of m-3M3FBS (Horowitz et al., 2005). In contrast m-3M3FBS caused activation of large conductance background-type potassium (LKbg) channels in B lymphocytes under the inside-out configuration suggesting that LKbg channels are regulated by a PLC-dependent mechanism (Nam et al., 2007).
In gastrointestinal smooth muscle, muscarinic receptor agonists produce excitation and contraction of smooth muscle (So and Kim, 2003) via activation of the PLC pathway through coupling between muscarinic receptors and Gq/11 proteins (Unno et al., 2003; Unno et al., 2006; Zholos, 2006). Therefore m-3M3FBS was tested on colonic smooth muscle to examine its effects on colonic excitability through a PLC-dependent pathway.
2. Materials and methods
2.1. Animals
Smooth muscle cells were prepared from colons removed from BALB/c mice. Mice were anesthetized with isoflurane and killed by cervical dislocation. Colons were removed from the animals through a midline abdominal incision. The animals were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
2.2. Mechanical responses
Segments of the proximal colon from 1 cm distal to the ileocecal sphincter were removed through a midline abdominal incision and opened along the mesenteric border. Luminal contents were removed by washing with Krebs–Ringer bicarbonate solution (KRB), and the cleaned tissue sheets were pinned down onto a Sylgard base with the mucosa facing up. The mucosa was removed leaving the tunica muscularis and remnants of the submucosa.
Mechanical responses were performed using standard organ-bath techniques. Strips of muscle (10 × 5 mm) were cut from the tunica muscularis by sharp dissection. The muscles were attached with sutures to a fixed mount within the organ bath and to an isometric strain gauge (World Precision Instruments, Sarasota, FL, U.S.A.). The muscles were immersed in oxygenated KRB and maintained at 37.5±0.5°C. The muscles were set at resting tension by applying 0.1–0.3 g of basal tension and then allowed to equilibrate for 1–2 hours with constant perfusion with fresh KRB. Contractions of the muscles were monitored, digitized and stored using Axoscope software (Axon Instruments, CA, USA). Contractions were quantified by calculations of area above the baseline using the pClamp software (v8.1, Axon instruments, CA, USA). The area under the curve (AUC) was determined as the integral values above the baseline of a selected area for 5 min recordings (g*min). The AUC for the tissues exposed to tested drugs were compared to the AUC for tissues under control conditions during an equivalent period of time. Drugs were diluted to the desired concentrations and applied to the muscles by switching the perfusion to the drug-containing solution.
2.3. Intracellular microelectrode recordings
After removing the mucosa, strips of proximal colon (1 cm in length × 0.5 cm in width) were taken from the region 1–2 cm from the ileocecal sphincter and pinned to a Sylgard (Dow Corning Corp., Midland, MI, USA) elastomer-coated recording chamber with the mucosal side of the circular muscle facing upward. Smooth muscle cells were impaled with glass microelectrodes filled with 3M KCl and having electrical resistances of 80–100 MΩ. Transmembrane potentials were measured with a standard high input impedance amplifier (WPI Duo 773, Sarasota, FL, USA). Electrical signals were recorded by a PC-style computer running AxoScope data acquisition software (Axon Instruments, CA, USA) and analyzed by Clampfit (v9.02, Axon Instruments, CA, USA). All experiments were performed in the presence of wortmannin (10μM) to reduce movement and facilitate impalements of cells for extended periods of time.
2.4. Preparation of isolated colonic myocytes
Colons were cut open along the longitudinal axis, pinned out in a Sylgard-lined dish, and washed with Ca2+-free phosphate-buffered saline containing (mM): 125 NaCl, 5.36 KCl, 15.5 NaHCO3, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose, and 11 HEPES and adjusted to pH 7.4 with NaOH. Mucosa and submucosa were removed with fine-tipped forceps. Pieces of muscle were incubated for 30–40 minutes at 37°C in a Ca2+-free solution (ml) containing 2 mg collagenase (Worthington Biochemical, Lakewood, NJ), 4 mg trypsin inhibitor, 4 mg fatty acid-free bovine serum albumin, 1 mg papain and 0.3mg dithiothreitol (Sigma-Aldrich, MO, USA). After enzymatic treatment, the muscles were washed with Ca2+-free solution and agitated gently to create a cell suspension. Dispersed smooth muscle cells were stored at 4°C in Ca2+-free solution. Cells were transferred from the refrigerator to the recording chamber. Drops of the cell suspensions were placed on the bottom of a 300 μl chamber mounted on an inverted microscope and allowed to adhere to the bottom of the chamber for 5 minutes before recording.
2.5. Voltage-clamp methods
Whole cell voltage-clamp techniques were used to record membrane currents from dissociated smooth muscle cells. Membrane currents were amplified by an Axopatch 1D (Axon Instruments, Foster City, CA) and digitized with an analog-to-digital converter (Digidata 1200, Axon Instruments). Data were collected at 5 kHz, filtered at 2 kHz via Bessel filter, and digitized online with pCLAMP software. The data were analyzed with the use of Clampfit software (version 9.2, Axon Instruments, CA, USA). Pipette resistances were 1–4 MΩ. The linear leak current was subtracted digitally. Conventional and perforated whole cell patch-clamp techniques were used for recording ionic currents under voltage clamp. For perforated patches, amphotericin B (60 mg/ml) was dissolved in DMSO, sonicated, and diluted in the pipette solution to give a final concentration of 270 μg/ml. Experiments were performed at room temperature (between 22 and 25°C).
2.6. Solutions
In order to measure inward currents, colonic myocytes were bathed in a Ca2+-containing physiological salt solution (CaPSS) containing (mM): 135 NaCl, 5 KCl, 2 CaCl2, 1.2 MgCl2, 10 glucose, 10 HEPES adjusted to pH 7.4 with Tris. The pipette solution for the study of inward currents contained (in mM): 135 CsCl, 0.1 EGTA, 0.1 Na2GTP, 3 MgATP, 10 glucose, 2.5 creatine phosphate disodium and 10 HEPES. This solution was adjusted to pH 7.2 with Tris. When studying outward currents, cells were perfused in MnPSS (same as CaPSS except Ca2+ was replaced with Mn2+ (2 mM)) and the pipette solution contained (in mM): 135 KCl, 10 BAPTA, 0.1 Na2GTP, 3 MgATP, 10 glucose, 2.5 creatine phosphate disodium and 10 HEPES and was adjusted to pH 7.2 with Tris.
2.7. Calcium imaging analysis
A stock solution of Fluo-4 AM (FluoroPure AM; Molecular Probes, Eugene, OR, USA) was dissolved in DMSO (50 μg Fluo-4 AM in 10 μl DMSO). One microlitre of Fluo-4 stock solution (5 μg) was added to dispersed colonic myocytes in 1 ml of Ca2+ free solution. Cells were incubated in Fluo-4 for 15 minutes (room temperature) after which they were perfused with CaPSS solution at 24 ± 0.5°C for 10 minutes to allow for de-esterification of the dye. Cells were imaged under an inverted fluorescence microscope (Nikon, TE2000-S; Technical Instruments, Burlingame CA, USA), using excitation and emission suitable for Fluo-4 (excitation 460–490 nm and emission > 515 nm), delivered via a xenon arc from a Lambda DG-5 (Sutter Instruments, Novato, CA, USA). Neutral density filters were used to adjust excitation intensity. Images of Ca2+-induced fluorescence changes were recorded at room temperature using a Hamamatsu ORCA digital camera (Bridgewater, NJ, USA) and SIMPLE PCI (version 5.3.1; Compix Inc. Imaging Systems, PA, USA). Fluorescence images were stored as a time series (one frame per sec). During patch experiments, standard ramp protocols were applied and voltage-dependent changes in [Ca2+]i recorded. Changes in fluorescence are shown relative to the baseline fluorescence (ΔF/F), where F denotes baseline fluorescence and ΔF is the change in fluorescence in response to stimulation by ramp depolarizations and the compounds m-3M3FBS and o-3M3FBS.
2.8. Statistical analysis
Data are reported as means ± S.E.M. In describing the voltage-clamp results, n is the number of tissues and cells tested. Statistical significance was evaluated by Student’s t test. P values less than 0·05 were considered significant.
2.9. Chemicals
N-(3-trifluoromethylphenyl)-2,4,6-trimethylbenzenesulfonamide (m-3M3FBS) and 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione (U-73122) were obtained from Calbiochem (San Diego, CA, USA). 2,4,6-Trimethyl-N-[2-(trifluoromethyl)phenyl]benzenesul fonamide (o-3M3FBS) was obtained from Tocris Bioscience (Ellisville, MO, USA). 4-aminopyridine (4-AP), tetraethylammonium chloride (TEA), tetrodotoxin (TTX) and wortmannin were obtained from Sigma (St. Louis, MO, USA).
3. RESULTS
3.1. m-3M3FBS inhibits contractility of murine colonic smooth muscle
We performed isometric force measurements to examine the effects of m-3M3FBS on murine colonic smooth muscle. Since excitatory and inhibitory neurotransmitters contribute to spontaneous phasic contractions (Wang et al., 2000), in all contractile experiments muscle strips were incubated with TTX (1μM), which blocks axonal action potential transmission and neurotransmitter release from nerve terminals. Figure 1A shows that after administration of TTX there was a significant increase in the amplitude of the spontaneous phasic contractions, indicating that inhibitory neurotransmitters may be dominant in murine colonic smooth muscle. Although the cellular basis of the rhythmic activities of the colon has not yet been clearly identified, the cyclic depolarizations from pacemaker cells (interstitial cells of Cajal) may evoke action potentials in smooth muscle cells and induce spontaneous colonic motility (Alberti et al., 2005).
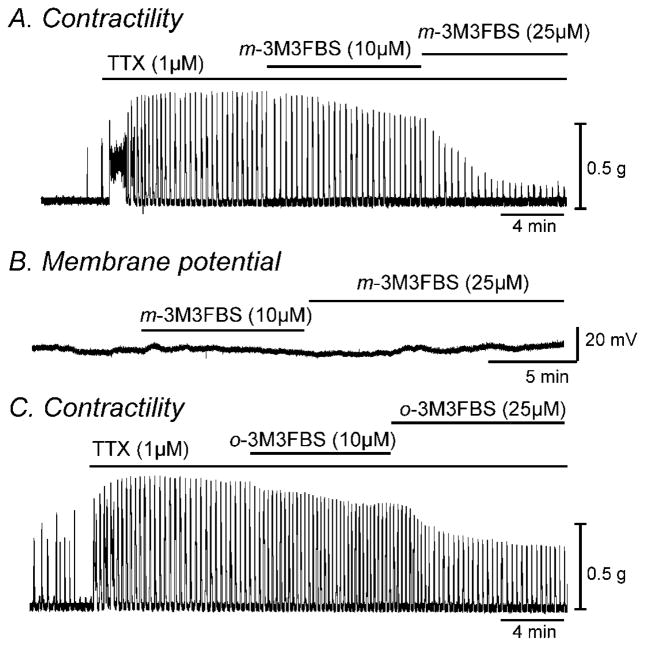
Fig. 1.
m-3M3FBS decreased contractility of colonic smooth muscle but had no effect on membrane potential. (A & C) Representative mechanical traces show that exposure to increasing concentrations of m-3M3FBS and o-3M3FBS (10 and 25μM) decreased the contractile responses in colonic smooth muscle in the presence of TTX (1μM). (B) Representative trace illustrating the lack of effect of m-3M3FBS (10 and 25μM) on membrane potential of intact murine colonic smooth muscle.
Contractile responses to m-3M3FBS were compared by determining the AUC (see materials and methods section). The AUC for 5 minute recordings was 526±62 g*min in control and 355±48 g*min in m-3M3FBS (n=10, P<0.001). Increasing the concentration of m-3M3FBS to 25μM caused a further decrease in AUC to 220±31 g*min (n=10, P<0.001 compared to 10μM, Fig. 1A). These effects of m-3M3FBS were reversible upon washout. Since changes in contractility seen by m-3M3FBS could be due to hyperpolarization, we performed membrane potential measurements using conventional microelectrode recordings. The application of m-3M3FBS (10 and 25μM) had no significant effect on membrane potential (n=5, Fig. 1B). We also examined the effect of o-3M3FBS on contractility of colonic smooth muscle. o-3M3FBS (10μM) decreased AUC from 1058±189 g*min in control (in the presence of TTX) to 840±134 g*min (n=4, P<0.05). Higher concentration of o-3M3FBS (25μM) further decreased AUC to 457±59 g*min (n=4, P<0.001 compared to 10μM, Fig. 1C). Therefore we performed patch clamp experiments to understand the mechanisms of the electrical and contractile responses seen by application of m-3M3FBS and o-3M3FBS.
3.2. m-3M3FBS inhibits delayed rectifier K+ currents in a PLC-independent manner
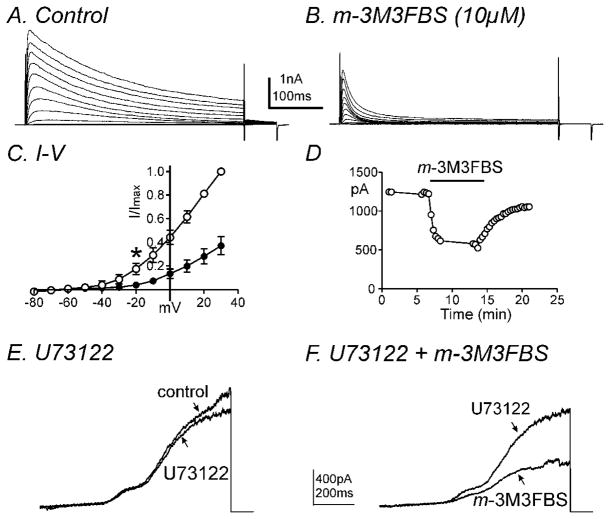
Since both m-3M3FBS and o-3M3FBS decreased contractility and m-3M3FBS did not affect membrane potential, we investigated PLC-dependent mechanisms which can affect down-stream signaling pathways following PLC activation (e.g. IP3-dependent Ca2+ release, diacylglycerol, protein kinase C, etc) on outward and inward currents. Firstly, we performed conventional dialyzed whole cell experiments to exclude the involvement of intracellular signaling pathways. The external solution was MnPSS and cells were dialyzed with K+-rich solution containing 10mM BAPTA. Ca2+ was replaced with Mn2+ (2mM) externally and decreased intracellular Ca2+ concentration using high concentration of BAPTA internally to minimize contamination by large-conductance Ca2+-activated K+ currents (BK). m-3M3FBS (10μM) significantly decreased net delayed rectifier K+ currents (IDR) during step depolarizations from −80mV to +30mV at a holding potential of −80mV (Fig. 2A & B). The averaged currents before and after m-3M3FBS (10μM) application at +20mV were 1327±460pA and 508±256pA, respectively (n=4, P<0.05). Figure 2C shows the normalized current-voltage (I-V) relationship with peak currents at +30mV. Peak IDR currents were significantly reduced at potentials positive to −20 mV (n=4, P<0.05). When colonic myocytes were repetitively depolarized to 0mV from a holding potential of −80mV every 20 seconds, the effect of m-3M3FBS on IDR was reversible (Fig. 2D). Furthermore we tested the effects of m-3M3FBS in the presence of the PLC inhibitor, U73122. Upon ramp depolarizations from −80mV to +80mV, U73122 (2μM) itself decreased IDR but not significantly (n=4, P=0.4, Fig. 2E). Additional application of m-3M3FBS (10μM) in the presence of U-73122 further decreased IDR from 591±83 pA to 244±100 pA (n=4, P<0.05, Fig. 2F). These data suggest that the effect of m-3M3FBS on IDR may not act through PLC activation.
Fig. 2.
m-3M3FBS decreased IDR in a PLC-independent manner. Membrane potential was stepped from −80 to +30mV in 10mV increments from a holding potential of −80mV in (A) control conditions and (B) in the presence of m-3M3FBS. (C) Summary of normalized I-V relationships in control (○) and m-3M3FBS (●). Peak currents (I) were normalized with the peak current at +30mV (Imax). m-3M3FBS (10μM) significantly decreased IDR (n=4, * denotes P<0.05 at −20mV). All tested potentials positive to −20mV were significant. (D) Time course of inhibition of IDR generated by repetitive step depolarizations to 0mV from a holding potential of −80mV every 20 seconds before and after m-3M3FBS application. (E & F) Representative current traces are shown of ramp depolarizations stepping from −80mV to +80mV every 30 seconds in the presence of (E) U73122 (2μM) and (F) U73122 (2μM) and m-3M3FBS (10μM). m-3M3FBS decreased IDR in the presence of U73122.
Therefore, we examined effects of o-3M3FBS on IDR. There was a significant decrease in the averaged currents before and after o-3M3FBS (10μM) at +20mV (784±126pA and 505±115pA, respectively (n=7, P<0.05, data not shown). At +20mV, there was a 69±9 % reduction in IDR in the presence of m-3M3FBS (10μM, n=4) and a 36±9 % reduction in IDR in the presence of o-3M3FBS (10μM, n=7).At the same concentration, m-3M3FBS was significantly more effective at reducing IDR than o-3M3FBS (P<0.05).
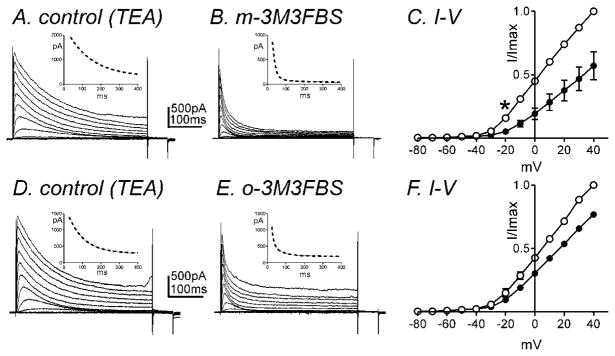
In a previous report, we found that IDR consists of two types of currents: A-type K+ currents (IA) and slowly-activating delayed rectifier K+ currents (IDRS) in murine colonic myocytes and these currents can be isolated pharmacologically (Koh et al., 1999b). In order to isolate IA, we added TEA (10mM) externally which inhibits IDRS (Koh et al., 1999b). Figure 3A shows representative traces of IA in the presence of TEA using the same protocol described previously. The application of m-3M3FBS (10μM) decreased IA (Fig. 3A & B). The normalized I-V relationship is shown in figure 3C with peak currents at +40 mV. The effects on IA were statistically significant at potentials positive to −20mV (n=4, P<0.05, Fig. 3C). o-3M3FBS (10μM) also decreased IA (n=4 Fig. 3D–F). At 0mV, there was a 57±10 % reduction in IA in the presence of m-3M3FBS (10μM, n=4) and a 29±2 % reduction in IA in the presence of o-3M3FBS (10μM, n=4). m-3M3FBS was significantly more effective than o-3M3FBS in reducing IA (n=4, P<0.05). Both compounds did not affect the voltage dependence of activation (e.g. V1/2 = −5.8±0.9mV in control, 2.1±3.2mV in the presence of m-3M3FBS). However, the time constant of inactivation at +20mV in the presence of TEA was 166±61 ms with a single exponential fit. The application of m-3M3FBS (10μM) induced fast inactivation (τf=11±2 and τs=54±12ms) with double exponential fit (see inset in Fig. 3A & B). o-3M3FBS (10μM) also significantly increased the rate of the fast component of inactivation from τ =83±6ms in control (presence of TEA) to τf =12±2ms and τs=70±17ms at +20mV (see inset in Fig. 3D & E).
Fig. 3.
IA is inhibited by m-3M3FBS. Membrane potential was stepped from −80 to +40mV in 10mV increments from a holding potential of −80mV (A) in the presence of TEA (10mM, control) and (B) in the presence of m-3M3FBS (10μM) in TEA. (C) Normalized I-V relationships in control (○) and m-3M3FBS (●). Peak currents (I) were normalized with the peak current at +40mV (Imax). m-3M3FBS (10μM) significantly decreased IA (n=4, * denotes P<0.05 at −20mV). All tested potentials positive to −20mV were significant. (D–F) o-3M3FBS also significantly decreased IA (n=4). Insets illustrate the time constant of inactivation with single exponential fit (panel A & C, dotted line) and double exponential fit (panel B & E, dotted line) at +20 mV.
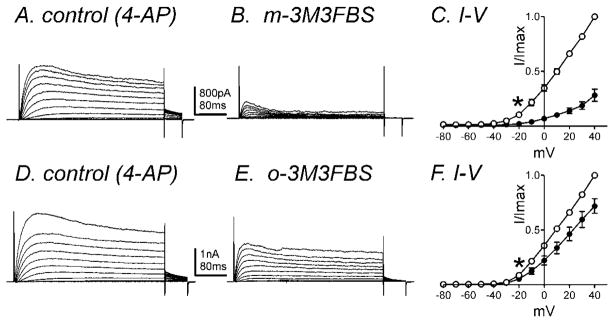
In order to confirm the effects of m-3M3FBS on IDRS, we performed experiments in the presence of 4-AP (5mM) which can inhibit A-type currents (Koh et al., 1999b). Figure 4A shows representative control traces of IDRS in the presence of 4-AP in the bath solution using the same protocol as previously described. Application of m-3M3FBS (10μM) decreased IDRS (Fig. 4A & B). The peak current in control and in the presence of m-3M3FBS was 334±86pA and 67±20pA at 0mV respectively (n=4, P<0.05). The normalized I-V relationship is shown in figure 4C. o-3M3FBS (10μM) also caused a significant decrease in IDRS from 431±52 to 293±127pA at 0mV (n=6, P<0.05, Fig. 4D–4F). There was 81±3% reduction of IDRS by m-3M3FBS compared with a 38±11% reduction by o-3M3FBS. m-3M3FBS was significantly more effective than o-3M3FBS at reducing IDRS (n=4, P<0.05).
Fig. 4.
IDRS is decreased by m-3M3FBS. Membrane potential was stepped from −80 to +40mV in 10mV increments from a holding potential of −80mV (A) in the presence of 4-AP (5mM, control) and (B) in the presence of m-3M3FBS (10μM) in 4-AP. (C) Normalized I-V relationships in control (○) and m-3M3FBS (●). Peak currents (I) were normalized with the peak current at +40mV (Imax). m-3M3FBS (10μM) significantly reduced IDRS (n=4, * denotes P<0.05 at −20mV). (D–F) o-3M3FBS also decreased IDRS (n=6, * denotes P<0.05 at −20mV). All tested potentials positive to −20mV were significant.
3.3. m-3M3FBS and o-3M3FBS inhibit inward currents
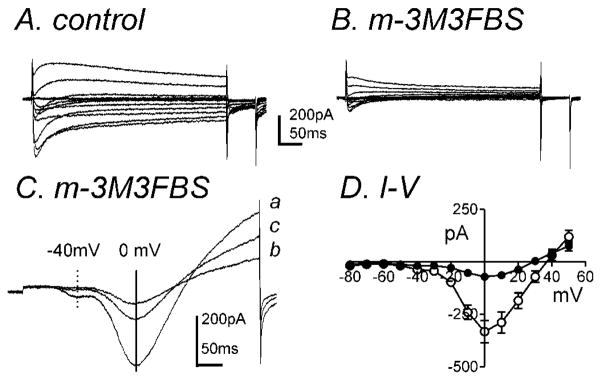
The inhibition of IDR should increase the contractile response with membrane depolarization since IA regulates the resting membrane potential (RMP). However m-3M3FBS decreased the contractile response without affecting the RMP. Therefore we examined the potential non-specific effects of m-3M3FBS on inward currents. The perforated configuration of the patch-clamp technique was used on freshly dispersed colonic myocytes in order to prevent run-down and preserve intracellular signaling pathways. In the pipette solution, KCl (140mM) was replaced with equimolar CsCl in order to isolate inward currents (see materials and methods). In a previous report, two types of inward currents have been identified in murine colonic myocytes, which are a low threshold voltage-activated Ca2+-current (ILVA) and a high threshold voltage-activated Ca2+ current (IL; L-type) (Koh et al., 2001). m-3M3FBS (10μM) significantly decreased inward currents during step depolarizations from −80mV to +50mV from a holding potential of −80mV (Fig. 5A & B). During ramp depolarizations from −80mV to +80mV over 500ms every 1 minute, two distinct components of the Ca2+ current were observed: ILVA peaked at ~−40mV (−27±10pA, n=5, see dashed line in Fig. 5C) and IL was recorded which peaked at approximately 0mV (195±30pA, see solid line in Fig. 5C). Inhibition of ILVA and IL by m-3M3FBS was partially reversible upon washout (Fig. 5C). The averaged I-V relationship is shown in figure 5D. m-3M3FBS (10μM) reduced ILVA from −37±10pA to −18±7pA at −40mV but not significantly (n=7, P=0.1). IL was significantly reduced from −332±53pA in control to −71±10pA in the presence of m-3M3FBS at 0mV (n=7, P<0.001, Fig. 5D). There was no significant difference in half activation and inactivation voltages before and during m-3M3FBS application (data not shown).
Fig. 5.
m-3M3FBS significantly decreased IL. Membrane potential was stepped from −80 to +50mV in 10mV increments from a holding potential of −80mV in (A) control and (B) in the presence of m-3M3FBS (10μM). (C) Representative traces where ramp depolarizations were applied from −80mV to +80mV over 500ms every 1 minute in control (a), m-3M3FBS (b), and washout (c). m-3M3FBS decreased peak ILVA (dashed line at −40mV and IL (vertical solid line at 0mV). (D) Summary of I-V relationships in control (○) and m-3M3FBS (●). m-3M3FBS (10μM) significantly decreased IL (n=7) but not ILVA.
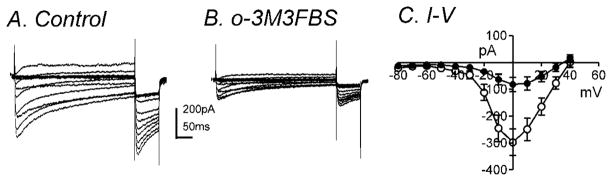
o-3M3FBS (10μM) significantly decreased IL during step depolarizations from −80mV to +40mV at a holding potential of −80mV (Fig. 6A & B). The averaged currents before and during o-3M3FBS (10μM) application at 0mV were −297±50pA and −82±26pA, respectively (n=7, P<0.001). Figure 6C shows the summarized I-V relationship with peak currents at 0mV (n=7). Analysis of the half activation and inactivation voltages before and during o-3M3FBS application revealed no significant differences (data not shown). At 0mV, there was a 77±3 % reduction in IL in the presence of m-3M3FBS (10μM, n=7) and a 70±8 % reduction in IL in the presence of o-3M3FBS (10μM, n=7). There was no significant difference between the percent reductions of IL by these two compounds.
Fig. 6.
o-3M3FBS significantly decreased IL. Membrane potential was stepped from −80 to +50mV in 10mV increments from a holding potential of −80mV (A) in control and (B) in the presence of o-3M3FBS (10μM). (C) Summarized I-V relationships show significant inhibition by o-3M3FBS on IL (n=7). (○) and (●) denote control and o-3M3FBS, respectively.
3.4. The effect of m-3M3FBS on IL is PLC-independent
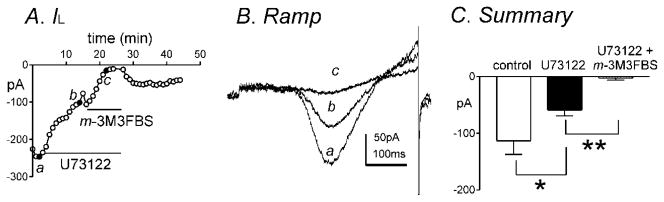
Since o-3M3FBS suppressed IL, this suggests that both m-3M3FBS and o-3M3FBS act in a PLC-independent manner. In order to further elucidate if the effects of m-3M3FBS were PLC-independent, cells were pretreated with the PLC inhibitor U73122 (1μM). U73122 significantly decreased peak IL from −113±24pA to −59±10pA at approximately 0mV (n=7, P<0.05, Fig. 7A–C). m-3M3FBS (10μM) caused a further decrease in IL from −59±10pA in the presence of U73122 (1μM) to −3±4pA at 0mV (n=7, P<0.001, Fig. 7A–C) therefore suggesting a PLC-independent mode of inhibition of IL by m-3M3FBS.
Fig. 7.
The PLC inhibitor, U73122, does not prevent m-3M3FBS-induced inhibition of IL. (A) Peak IL by U73122 (1μM) and both U73122 (1μM) and m-3M3FBS (10μM) was plotted as a function of time. (B) Representative traces where ramp depolarizations were applied from −80mV to +80mV over 500ms every 1 minute in (a) control, (b) in the presence of U73122 and (c) in the presence of U73122 and m-3M3FBS. (C) Summary of peak IL at 0 mV by U73122 (1μM) and both U73122 (1μM) and m-3M3FBS (10μM) (n=7). * and ** denote P<0.05 and P<0.001, respectively.
3.5. m-3M3FBS and o-3M3FBS stimulate [Ca2+]i
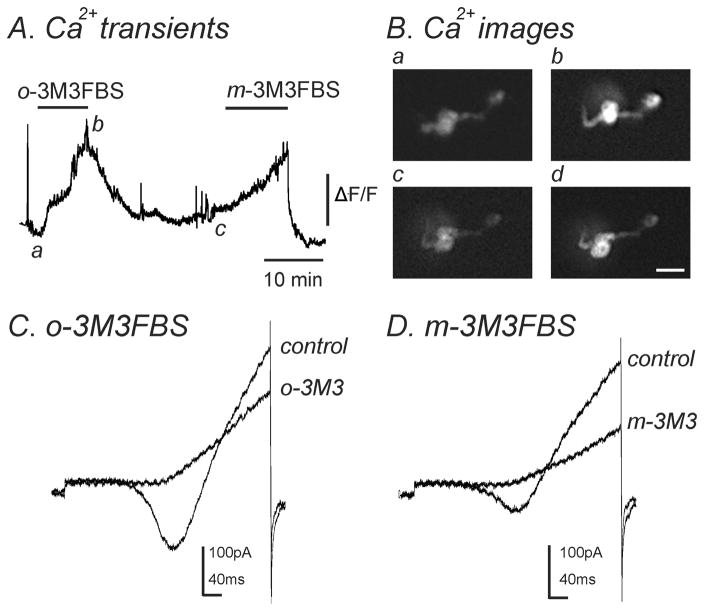
Previous reports have found that m-3M3FBS can cause an increase in [Ca2+]i in a number of different cell types (Bae et al., 2003; Krjukova et al., 2004). To determine if m-3M3FBS could evoke a similar response in murine colonic myocytes, we measured intracellular Ca2+ transients in conjunction with the patch clamp technique to analyze changes in [Ca2+]i along with simultaneous changes in inward currents. The application of m-3M3FBS (10–25μM) gradually increased [Ca2+]i over a 10 minute period (n=7, Fig. 8A, Bc & Bd) and simultaneously decreased IL (n=7, Fig. 8D). o-3M3FBS (10–25μM) also caused an increase in [Ca2+]i as well as simultaneously decreased IL (n=4, Fig. 8A, Ba & Bb, C).
Fig. 8.
Both m-3M3FBS and o-3M3FBS increased [Ca2+]i and simultaneously decreased IL. (A) Representative trace showing an increase in [Ca2+]i upon application of o-3M3FBS (10μM) which was reversible upon washout. Addition of m-3M3FBS (10μM) to the same cell also increased [Ca2+]i which was reversible. (B) Images of a single smooth muscle cell loaded with Fluo-4AM. These images correspond to [Ca2+]i measurements shown in panel A during (a) control conditions, (b) in the presence of o-3M3FBS (10μM), (c) after washout of o-3M3FBS and (d) in the presence of m-3M3FBS (10μM). Scale bar= 20μm. (C & D) During [Ca2+]i measurements, a single ramp depolarization was applied from −80mV to +80mV over 500ms in (C) control conditions and in the presence of o-3M3FBS (o-3M3; 10μM) and (D) after washout of o-3M3FBS and in the presence of m-3M3FBS (m-3M3; 10μM). Both o-3M3FBS (n=4) and m-3M3FBS (n=7) inhibited IL.
4. DISCUSSION
Many studies have implicated PLC as a vital component in carrying out important cellular functions such as apoptosis, proliferation and differentiation (Rebecchi and Pentyala, 2000; Rhee, 2001; Hicks et al., 2008; Suh et al., 2008). The identification of a novel PLC agonist, m-3M3FBS was an exciting finding since it could be used in a direct way to ascertain the exact roles of PLC in contractility and ion channel regulation. m-3M3FBS induces the formation of inositol phosphates in U937 cells thus suggesting stimulation of PLC activity (Bae et al., 2003).
In gastrointestinal smooth muscle, acetylcholine and carbachol produce excitation and contraction of smooth muscle via stimulation of muscarinic receptors that are coupled to Gq/11 proteins resulting in activation of PLC-β (Unno et al., 2003; Unno et al., 2006; Zholos, 2006). The important role of PLC in this pathway has been illustrated as application of the PLC-blocker, U73122 prevents cation currents activated by carbachol that are essential to cause depolarization, subsequent activation of voltage-dependent Ca2+ channels resulting in contraction (Okamoto et al., 2004). Since carbachol activates the PLC pathway, direct PLC activation using m-3M3FBS would be expected to cause a similar depolarization and contraction. However we found m-3M3FBS and o-3M3FBS actually decreased colonic contractility. A possible explanation could be through activation of K+ channels and/or inhibition of Ca2+ influx through non-selective cation channels or Ca2+ channels thus causing hyperpolarization of RMP and decreased contractility. However m-3M3FBS did not significantly change the membrane potential. Since none of the tissue experiments could explain the PLC-mediated effects of m-3M3FBS, it was essential to examine what channels are affected by m-3M3FBS through patch clamp studies.
Many studies have examined the role of PLC and phosphatidylinositol 4,5-bisphosphate (PIP2) in ion channel regulation. KCNQ currents are known to require PIP2 for activation since PIP2 depletion by the muscarinic receptor agonist oxotremorine-M and m-3M3FBS caused inhibition of these currents (Horowitz et al., 2005). Menthol-evoked TRPM8 (Transient receptor potential cation channel, subfamily M, member 8) currents were reduced in HEK293T cells by m-3M3FBS (Daniels et al., 2009). On the other hand, stretch-activated PLC can cause LKbg activation through degradation of PIP2. This effect was mimicked by application of m-3M3FBS (Nam et al., 2007). TRPA1 (Transient receptor potential cation channel, subfamily A, member 1) currents were potentiated by m-3M3FBS in dorsal root ganglia neurons (Wang et al., 2008). In general there are no critical experiments that have examined potential PLC-independent effects of m-3M3FBS on native ion channels.
Firstly, we examined the effects of m-3M3FBS and o-3M3FBS on IDR in murine colonic myocytes and found that both compounds decreased IDR. m-3M3FBS was significantly more effective at reducing IDR than o-3M3FBS. This difference could be due to slight structural variation between these two compounds. It has previously been reported that IDR is not affected by U73122 in murine atrial myocytes (Cho et al., 2001). We found that U73122 did not significantly decrease IDR in colonic myocytes. However, preincubation with this PLC inhibitor did not prevent inhibition of IDR by m-3M3FBS. Since the PLC-pathway is not expected to be conserved in dialyzed whole cell configurations, this suggests the effects of m-3M3FBS on IDR could be non-specific.
IDR are composed of IDRS and IA (Koh et al, 1999b). IA are resistant to TEA and IDRS are resistant to 4-AP. Therefore, we isolated IA with treatment of TEA (10mM). m-3M3FBS decreased IA with induction of fast inactivation. In previous reports, Ca2+-calmodulin-dependent protein kinase II (CaMKII) regulated inactivation kinetics of IA. CaMKII inhibitors increased the rate of inactivation whereas increasing [Ca2+]i decreased the rate of inactivation (Koh et al, 1999a; Amberg et al., 2001; Sergeant et al., 2004). Our findings (see Fig. 8) and previous reports (Bae et al., 2003; Krjukova et al., 2004; Roedding et al., 2006) demonstrate that m-3M3FBS can increase [Ca2+]i. An increase in [Ca2+]i would be expected to decrease the rate of inactivation of IA. However m-3M3FBS and o-3M3FBS increased the rate of inactivation of IA suggesting these compounds may have unspecific effects on the inactivation kinetics of IA.
Inhibition of IDRS increases the amplitude of spike action potential in murine colon (Koh et al., 1999b). Therefore, we expect an increase in contractility upon treatment of both m-3M3FBS and o-3M3FBS since both drugs inhibit IDRS. However the contractility was decreased by these agents. In addition, a previous study showed that IA is involved in the regulation of the RMP and inhibition of this conductance induces depolarization with continuous spike activity in murine colon (Koh et al., 1999b). Since the RMP is not changed by m-3M3FBS, further experiments are needed to elucidate how membrane potential is not changed by application of m-3M3FBS to colonic smooth muscle.
In a previous report, two types of inward currents (ILVA and IL) have been identified in murine colonic myocytes (Koh et al., 2001). Our studies revealed that m-3M3FBS significantly decreased IL but not ILVA. The decreased contraction seen upon its application to colonic smooth muscle can be explained through inhibition of IL as this is the major source of Ca2+ required for contraction (Liu et al., 2001; Wegener et al., 2006). These effects could be via a PLC-dependent mechanism. However o-3M3FBS also showed similar results as m-3M3FBS suggesting that the effect on IL could be PLC-independent. This data suggests that the common structures shared by both compounds can directly bind and inhibit these channels in a non-specific manner. However, further experiments need to be performed to identify the specific mechanisms of inhibition of these compounds on these channels.
In ion channel studies, the selectivity of the PLC inhibitor U73122 has been disputed (Mogami et al., 1997; Walker et al., 1998; Hughes et al., 2000; Horowitz et al., 2005; Takenouchi et al., 2005; Sickmann et al., 2008; Klose et al., 2008). It has been shown that U73122 revealed PLC-independent suppression of G protein-coupled inward rectifier K+ channels (Sickmann et al., 2008) and BK (Klose et al., 2008). Furthermore, U73122 caused a slow rise in [Ca2+]i and a total block of L-type Ca2+ channels in NG108-15 cells (Jin et al., 1994). Our data also supports PLC-independent effects of U73122 since its application significantly decreased IL. In addition, pre-incubation with U73122 did not prevent a further reduction in IL by m-3M3FBS. Since o-3M3FBS proved to be as efficient as m-3M3FBS in decreasing IL, this data strongly suggests that the actions of m-3M3FBS are not attributable to its expected PLC-activation. The similar effects of m-3M3FBS and o-3M3FBS on IL suggest that the common structure they share can directly bind and inhibit these channels in an antagonistic manner.
Upon application of m-3M3FBS, an increase in [Ca2+]i has been a common finding in various cell lines (Bae et al., 2003; Krjukova et al., 2004; Roedding et al., 2006). For example, a gradual increase in [Ca2+]i was recorded after application of m-3M3FBS in human neuroblastoma cells and this increase was proposed to be due to mechanisms other than IP3 (Krjukova et al., 2004). Our studies showed that m-3M3FBS gradually increased [Ca2+]i but simultaneously inhibited IL. Since this trend was also found with application of o-3M3FBS, this suggests a PLC-independent mechanism of action causing this increase [Ca2+]i in an agonistic manner. These findings were different from the effects on the ionic currents in colonic smooth muscle which revealed antagonistic effects by both compounds. The increase in [Ca2+]i by these compounds would be expected to increase murine colonic contractility. However, we found that contractility was decreased in colonic strips. Therefore, the inhibition of IL by m-3M3FBS and o-3M3FBS could explain the reduction in contractile responses and thus may be the dominant source of [Ca2+]i required for contraction.
In conclusion, our studies report the novel and unexpected finding that m-3M3FBS and o-3M3FBS cause a decrease in contractility in murine colonic muscle. Both compounds acted as antagonists as they inhibited IL and IDR via mechanisms independent of PLC. Conversely, both compounds revealed agonist-like activities as they caused an increase in [Ca2+]i. Therefore, caution must be employed when interpreting their effects at the tissue and cellular level due to the non-specificity of these drugs.
Acknowledgments
This work has been supported by NIH/NIDDK PO1-DK41315.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberti E, Mikkelsen HB, Larsen JO, Jimenez M. Motility patterns and distribution of interstitial cells of Cajal and nitrergic neurons in the proximal, mid- and distal-colon of the rat. Neurogastroenterol Motil. 2005;17:133–147. doi: 10.1111/j.1365-2982.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Koh SD, Perrino BA, Hatton WJ, Sanders KM. Regulation of A-type potassium channels in murine colonic myocytes by phosphatase activity. Am J Physiol Cell Physiol. 2001;281:C2020–2028. doi: 10.1152/ajpcell.2001.281.6.C2020. [DOI] [PubMed] [Google Scholar]
- Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a Compound That Directly Stimulates Phospholipase C Activity. Mol Pharmacol. 2003;63:1043–1050. doi: 10.1124/mol.63.5.1043. [DOI] [PubMed] [Google Scholar]
- Cho H, Youm JB, Ryu SY, Earm YE, Ho WK. Inhibition of acetylcholine-activated K+ currents by U73122 is mediated by the inhibition of PIP2-channel interaction. Br J Pharmacol. 2001;134:1066–1072. doi: 10.1038/sj.bjp.0704347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, Takashima Y, McKemy DD. Activity of the Neuronal Cold Sensor TRPM8 Is Regulated by Phospholipase C via the Phospholipid Phosphoinositol 4,5 Bisphosphate. J Biol Chem. 2009;284:1570–1582. doi: 10.1074/jbc.M807270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden K, Sondek J. General and Versatile Autoinhibition of PLC Isozymes. Mol Cell. 2008;31:383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SA, Gibson WJ, Young JM. The interaction of U-73122 with the histamine H1 receptor: implications for the use of U-73122 in defining H1 receptor-coupled signalling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:555–558. doi: 10.1007/s002100000326. [DOI] [PubMed] [Google Scholar]
- Jin W, Lo TM, Loh HH, Thayer SA. U73122 inhibits phospholipase C-dependent calcium mobilization in neuronal cells. Brain Res. 1994;642:237–243. doi: 10.1016/0006-8993(94)90927-x. [DOI] [PubMed] [Google Scholar]
- Klose A, Huth T, Alzheimer C. 1-[6-[[(17β)-3-Methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122) Selectively Inhibits Kir3 and BK Channels in a Phospholipase C-Independent Fashion. Mol Pharmacol. 2008;74:1203–1214. doi: 10.1124/mol.108.047837. [DOI] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Ro S, Mason HS, Kenyon JL, Sanders KM. Novel voltage-dependent non-selective cation conductance in murine colonic myocytes. J Physiol. 2001;533:341–355. doi: 10.1111/j.1469-7793.2001.0341a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Perrino BA, Hatton WJ, Kenyon JL, Sanders KM. Novel regulation of the A-type K+ current in murine proximal colon by calcium-calmodulin-dependent protein kinase II. J Physiol. 1999a;517:75–84. doi: 10.1111/j.1469-7793.1999.0075z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Ward SM, Dick GM, Epperson A, Bonner HP, Sanders KM, Horowitz B, Kenyon JL. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J Physiol. 1999b;515(2):475–487. doi: 10.1111/j.1469-7793.1999.475ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krjukova J, Holmqvist T, Danis AS, Akerman KE, Kukkonen JP. Phospholipase C activator m-3M3FBS affects Ca2+ homeostasis independently of phospholipase C activation. Br J Pharmacol. 2004;143:3–7. doi: 10.1038/sj.bjp.0705911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001;120:480–489. doi: 10.1053/gast.2001.21167. [DOI] [PubMed] [Google Scholar]
- Mogami H, Mills CL, Gallacher DV. Phospholipase C inhibitor, U73122, releases intracellular Ca2+, potentiates Ins(1,4,5)P3-mediated Ca2+ release and directly activates ion channels in mouse pancreatic acinar cells. Biochem J. 1997;324:645–651. doi: 10.1042/bj3240645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Lee HS, Nguyen YH, Kang TM, Lee SW, Kim HY, Kim SJ, Earm YE, Kim SJ. Mechanosensitive activation of K+ channel via Phospholipase C-induced depletion of phosphatidylinositol 4,5-bisphosphate in B lymphocytes. J Physiol. 2007;582:977–990. doi: 10.1113/jphysiol.2007.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Unno T, Arima D, Suzuki M, Yan HD, Matsuyama H, Nishimura M, Komori S. Phospholipase C involvement in activation of the muscarinic receptor-operated cationic current in guinea pig ileal smooth muscle cells. J Pharm Sci. 2004;95:203–213. doi: 10.1254/jphs.fp0030635. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Pentyala SN. Structure, Function, and Control of Phosphoinositide-Specific Phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of Phosphoinositide-Specific Phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roedding AS, Li PP, Warsh JJ. Characterization of the transient receptor potential channels mediating lysophosphatidic acid-stimulated calcium mobilization in B lymphoblasts. Life Sci. 2006;80:89–97. doi: 10.1016/j.lfs.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Sickmann T, Klose A, Huth T, Alzeheimer C. Unexpected suppression of neuronal G-protein-activated, inwardly rectifying K+ current by common phospholipase C inhibitor. Neurosci Lett. 2008;436:102–106. doi: 10.1016/j.neulet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Ohya S, Reihill JA, Perrino BA, Amberg GC, Imaizumi Y, Horowitz B, Sanders KM, Koh SD. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2004;288:C304–C313. doi: 10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- So I, Kim WK. Nonselective Cation Channels Activated by the Stimulation of Muscarinic Receptors in Mammalian Gastric Smooth Muscle. J Smooth Muscle Res. 2003;39:231–247. doi: 10.1540/jsmr.39.231. [DOI] [PubMed] [Google Scholar]
- Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, Fukami K, Kataoka T, Yun S, Ryu SH. Multiple Roles of Phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Ogihara K, Sato M, Kitani H. Inhibitory effects of U73122 and U73343 on Ca2+ influx and pore formation induced by the activation of P2X7 nucleotide receptors in mouse microglial cell line. Biochim Biophys Acta. 2005;1726:177–186. doi: 10.1016/j.bbagen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Unno T, Kwon SC, Okamoto H, Irie Y, Kato Y, Matsuyama H, Komori S. Receptor signaling mechanisms underlying muscarinic agonist-evoked contraction in guinea-pig ileal longitudinal smooth muscle. Br J Pharmacol. 2003;139:337–350. doi: 10.1038/sj.bjp.0705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unno T, Matsuyama H, Okamoto H, Sakamoto T, Yamamoto M, Tanahashi Y, Yan HD, Komori S. Muscarinic cationic current in gastrointestinal smooth muscles: signal transduction and role in contraction. Auton Autacoid Pharmacol. 2006;26:203–217. doi: 10.1111/j.1474-8673.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Walker EM, Bispham JR, Hill SJ. Nonselective effects of the putative phospholipase C inhibitor, U73122, on adenosine A1 receptor-mediated signal transduction events in Chinese hamster ovary cells. Biochem Pharmacol. 1998;56:1455–1462. doi: 10.1016/s0006-2952(98)00256-1. [DOI] [PubMed] [Google Scholar]
- Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000;302:331–342. doi: 10.1007/s004410000272. [DOI] [PubMed] [Google Scholar]
- Wegener JW, Schulla V, Koller A, Klugbauer N, Feil R, Hofmann F. Control of intestinal motility by the Cav1.2 L-type calcium channel in mice. FASEB J. 2006;20:1260–1262. doi: 10.1096/fj.05-5292fje. [DOI] [PubMed] [Google Scholar]
- Zholos A. Regulation of TRP-like muscarinic cation current in gastrointestinal smooth muscle with special reference to PLC/InsP3/Ca2+ system. Acta Pharmacol Sin. 2006;27:833–842. doi: 10.1111/j.1745-7254.2006.00392.x. [DOI] [PubMed] [Google Scholar]