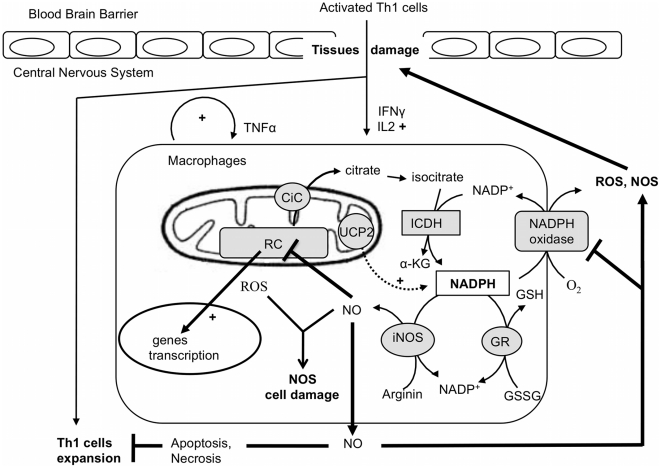
Figure 6. The redox balance in macrophages during inflammation induced by EAE.
Once activated, Th1 cells enter the CNS and produce proinflamatory cytokines (IFNγ, Il2) that stimulate resident macrophages. Inflammation is further increased with the autocrine production of TNFα by macrophages. At the cellular level, ROS production stimulates the expression of proinflammatory genes such as the NO synthase gene. In one hand, nitric oxide inhibits the respiratory chain (RC), which, in turn, increases the mitochondrial ROS production and consequently oxidative cell damage. In the other hand, NO prevent Th1 cell expansion and inhibits the NAPH oxidase enzyme, an essential ROS producer that trigger tissue damage and brain blood barrier destruction. At the molecular level, NADPH plays a pivotal function in the redox balance as substrate of the NADPH oxidase, the NO sytnhase (iNOS) and the gluthation reductase (GR). In this context, mitochondrial carriers, including UCP2, could modulate the availability of this substrate. For instance the citrate carrier supplies an additional source of cytosolic NADPH by exporting citrate that is further oxidized into alpha keto glutarat (α-KG) by the cytosolic isocitrate dehydrogenase (ICDH).