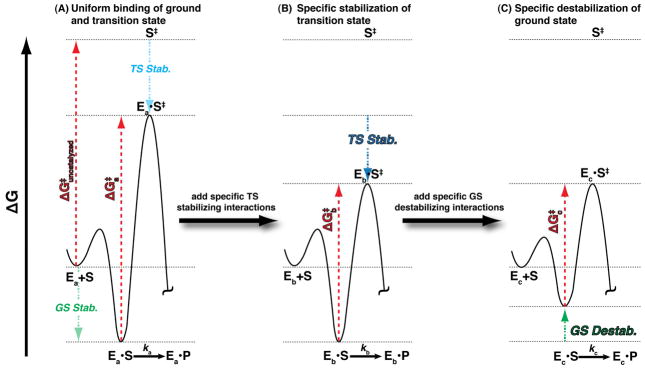
Figure 5.
Free energy reaction profiles illustrating how preferential E•S ground state destablization relative to the free E+S ground state and transition state can contribute to catalysis in the E•S → E•S‡ reaction step. (A) A hypothetical enzyme, Ea, that stabilizes the ground state (E•S) and transition state (E•S‡) equally, resulting in a reaction barrier, , equal to the uncatalyzed reaction barrier . Thus, this enzyme is not a catalyst. (B) Enzyme Eb is modified from Ea such that additional interactions are added that specifically stabilize the transition state relative to the E+S and E•S states. Thus, is less than and the rate constant kb is larger than ka (in Part A). This type of effect could arise from addition of a general acid or base catalyst for example. (C) Enzyme Ec destabilizes the ground state relative to the E+S and transition state such that is less than and the rate constant kc is larger than kb (in Part B).