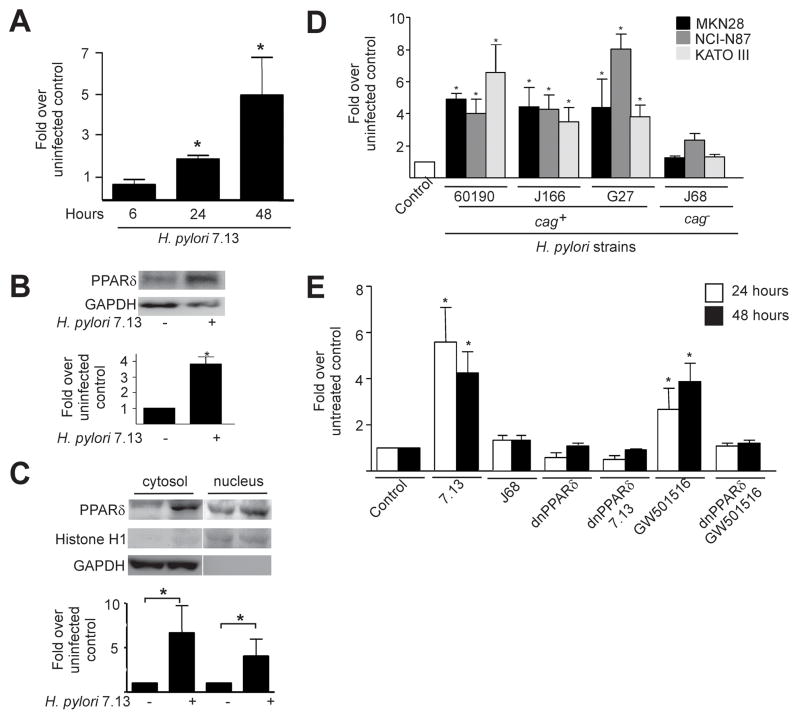
Figure 1. cag+ Helicobacter pylori induce expression and functional activation of PPARδ in gastric epithelial cells.
(A) MKN28 cells were co-cultured with H. pylori strain 7.13. RNA was analyzed in duplicate by real-time PCR. Data represent fold pparδ induction in infected versus uninfected cells from experiments performed on at least three occasions. Error bars, SEM for all panels. *p< .05 versus uninfected control. (B) MKN28 cells were co-cultured with strain 7.13. Forty-eight hours post-infection, whole cell lysates were analyzed by Western blot using an anti-PPARδ antibody. Anti-GAPDH antibody served as a normalization control. Densitometric analysis of Western blots performed on at least three occasions is shown below representative blots. *p< .05 versus uninfected control. (C) MKN28 cells were co-cultured with strain 7.13. Forty-eight hours post-infection, protein was analyzed by Western blot using an anti-PPARδ antibody. Anti-Histone H1 and anti-GAPDH antibodies served as normalization controls for nuclear and cytosolic fractionations, respectively. Densitometric analysis of Western blots performed on at least three occasions is shown below representative blots. *p< .05 versus uninfected control. (D) MKN28, NCI-N87 or KATO III cells were co-cultured with cag+ strains 60190, J166 or G27, or the cag− strain J68. RNA was extracted 24 hours post-infection and analyzed for pparδ expression. *p< .05 versus uninfected control. (E) MKN28 cells were co-transfected with PPRE3-tk-luciferase and pRL-SV40 with empty vector or dominant-negative PPARδ plasmids followed by treatment with strains 7.13 or J68, or the PPARδ agonist GW501516. Dual luciferase assays were performed as described in Methods. *p< .0004 versus untreated control.