Abstract
Previous studies have provided conflicting evidence regarding whether the magnocellular (M) or parvocellular (P) visual pathway is primarily responsible for triggering involuntary orienting. Here, we used event-related potentials (ERPs) to provide new evidence that both the M and P pathways can trigger attentional capture and bias visual processing at multiple levels. Specifically, cued-location targets elicited enhanced activity, relative to uncued-location targets, at both early sensory processing levels (indexed by the P1 component) and later higher-order processing stages (as indexed by the P300 component). Furthermore, the present results show these effects of attentional capture were not contingent on the feature congruency between the cue and expected target, providing evidence that this biasing of visual processing was not dependant on top-down expectations or within-pathway priming.
Keywords: involuntary, attention capture, ERP, P1, P3, P300
1. Introduction
As we navigate through our environment, we are only able to process a fraction of the information presented to our visual system. In some cases, highly salient stimuli will ‘pop out’ and win the competition for processing resources, with little influence from top-down mechanisms. These reflexive shifts of attention can briefly enhance visual processing with little if any volitional effort from the observer. Events with high feature salience, such as an abruptly appearing object (Jonides, 1981, Yantis and Jonides, 1984; Yantis and Hillstrom, 1994;), an abrupt luminance change (Posner, 1980; Atchley et al., 2000; Snowden, 2002; Theeuwes, 1995), or the onset of motion (Abrams & Christ, 2003; Folk et al., 1994; Guo et al., 2010) have been shown to capture attention and bias visual processing.
Attentional capture is often measured using behavioral measures such as reaction time and accuracy. Benefits of attentional capture are evidenced by faster reaction times and/or better accuracy to a target stimulus occurring at the same location as a preceding “cue” stimulus (i.e. cued-location target) relative to a target appearing in a different location from the preceding cue (i.e. uncued-location target), even when the cues are non-predictive of target location. These effects are transient, however, diminishing in magnitude with increasing time between cue and target (i.e., increasing stimulus onset asynchrony, SOA), and eventually being replaced by slower target reaction time at the cued location, a phenomenon referred to as inhibition of return (IOR; Posner & Cohen, 1984).
Event-related potentials (ERPs) have been used to measure the levels of neural processing that are affected by involuntary attentional capture. These studies have found that non-predictive cues modulate the neural processing of subsequent targets at multiple levels of information processing. At the short cue-to-target SOAs, when behavioral facilitation is typically found, the target-evoked P1 component is enhanced following a non-predictive cue stimulus at the same location (Fu, et al., 2001; 2005; Hopfinger and Mangun, 1998; 2001; Hopfinger & Ries, 2005). The P1 is the first major positive-voltage component evoked by upper visual field stimuli, and it is thought to arise from extrastriate cortex with a latency of ~80–120 msec. Cued-location targets also show an enhancement of higher order processing as indexed by the P300 component (more specifically the P3b). The P300/P3b is observed as a positivity over central posterior scalp sites at ~300 msec latency and is believed to represent aspects of information processing such as context updating and target stimulus processing (Polich, 2007). The amplitude of the P300 is positively correlated to stimulus relevance and the amount of attentional resources employed in a given task (Ruchkin et al., 1990; Wickens, et al., 1983).
Whereas neural processing is enhanced at multiple levels for cued-location targets when the cue-to-target interval is short (less than 300 msec), a different pattern emerges at longer SOAs. At SOAs longer than 500 msec, the P1 has been found to be significantly reduced for cued-location targets relative to uncued-location targets (Hopfinger & Mangun, 1998; McDonald et al., 1999; Prime & Ward, 2004). At these longer SOA’s, the P300 does not typically differ between cued and uncued location targets (Hopfinger and Mangun, 1998; 2001; Fu et al., 2001).
Although ERP and behavioral studies have shown that non-predictive cues can involuntarily capture attention and dynamically modulate the processing of subsequent stimuli, it is unknown which visual features of the cue stimuli trigger these effects on target processing. Specifically, there has been debate about whether the magnocellular (M-pathway) or the parvocellular (P-pathway) visual pathways trigger reflexive attention. The M and P pathways convey different information about the visual world up to and beyond the visual cortex (reviewed in Kaplan, 2004). The P-pathway is highly responsive to color stimuli and high spatial frequencies, and it is less sensitive to low luminance contrast than is the M-pathway (Kulikowski et al., 2002). The M-pathway has a fast conduction speed, favors stimuli that move and/or contain low luminance contrast, and is highly sensitive to low spatial frequencies, but is relatively insensitive to color.
Regarding attention, the M-pathway has been hypothesized to play a dominant role in reflexive orienting (e.g., Egeth & Yantis, 1997). For example, in a line motion illusion paradigm, Steinman et al. (1997) found that peripheral luminance cues that primarily stimulated the M-pathway produced larger attention effects than did isoluminant color cues that stimulated the P-pathway. Cheng et al. (2004) found that serial search became significantly slower when target stimuli were isoluminant with the surround (therefore being limited to P-pathway) compared to when they contain a contrast in luminance that stimulated the M-pathway, again suggesting the M-pathway plays a dominant role in attentional orienting. Khoe et al. (2005) found that translational motion of a surface triggers an exogenous shift of attention in favor of that surface with respect to an overlapping surface, even when color selection biases from the P-pathway are ruled out. A link between attention and the M-pathway has also been suggested by the finding that patients suffering from attentional neglect are more deficient in processing information from the M-pathway compared to the P-pathway (Pitzalis et al., 2005).
While the above research suggests that the M-pathway plays an important role in attentional allocation, it is still unclear what role the P-pathway plays in capturing attention. Recent evidence supports the idea that isoluminant color cues engage reflexive attention mechanisms and produce similar costs and benefits in behavior as those found in studies where cues primarily consist of a luminance contrast or M stream activation (Cole et al., 2005; Snowden, 2002). To prevent luminance from contributing to attentional capture, Snowden (2002) presented continuous random luminance noise in the background during a non-predictive peripheral cue/target paradigm. The results from this study suggested that the P-pathway can trigger reflexive visual orienting, as the non-predictive isoluminant color cues resulted in a facilitation of RTs to cued relative to uncued targets. However, the cue stimulus in this case consisted of the appearance of a new object, as well as a color change, and therefore, it was unclear if color alone captured attention. When Cole et al. (2005) conducted a similar study but with the addition of a condition including a color change to an existing object, only the new objects were found to facilitate reaction times. It was concluded that a unique color change alone cannot capture attention unless it also defines a new object. This conclusion was challenged, however, by Lu (2006) who conducted a similar experiment but varied the duration of the cue. Lu reasoned that since the parvocellular system has sluggish response the lack of capture may not have completely activated the P-pathway in the Cole et al study. Lu (2006) replicated the finding that there was no capture with a short 50ms duration cue, but found that attention was captured by a color change to an old object when the cue duration was 75, 100, 125, and 150ms. This finding suggests that reflexive attention can be triggered by the P system, but the stimulus triggering the capture must be presented long enough to robustly stimulate the P pathway.
Although these studies provide evidence that the M and P-pathways are capable of enhancing behavioral responses to cued-location targets in a similar fashion, it is unclear if these behavioral effects result from the same neural mechanisms. Previous ERP studies have shown that reflexive attention affects multiple levels of neural processing, but those studies used cues that likely stimulated both the M and P-pathways. The main aim of the present study was to investigate whether the M and P-pathways capture attention and modify all of the same levels of target processing in the brain, or whether some of these effects are triggered by only one pathway or the other. In addition, we examined whether these effects were dependent on top-down control settings (i.e., expecting the target to be an M or P-pathway stimulus). Research has shown that top-down attentional control settings can significantly affect behavioral measures of attentional capture (e.g., Folk et al, 1992; 1994), although ERP research has shown that capture effects at some stages of processing are unaffected by top-down settings (Hopfinger & Ries, 2005).
For the present study, we designed stimuli containing features that preferentially activated either the M or P pathway, and used these stimuli as cues and targets in a peripheral cueing paradigm while measuring ERPs and behavior. We used these stimuli in a pair of experiments in order to test for any differential effects between P and M cues. In both experiments, cues could be either “P cues” or “M cues”. In Experiment 1, the target was always a red target (i.e., a “P target”); whereas in Experiment 2, the target was always a motion target (i.e., an “M target”).
2. Methods. Experiment 1: P-pathway targets
2. 1. Participants
Participants consisted of 16 healthy, right-handed adults (average age 26.2 years; 5 females) who were reimbursed $10 per hour for their time. Before participating in any procedure, each participant gave informed written consent that was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
2.2. Procedure
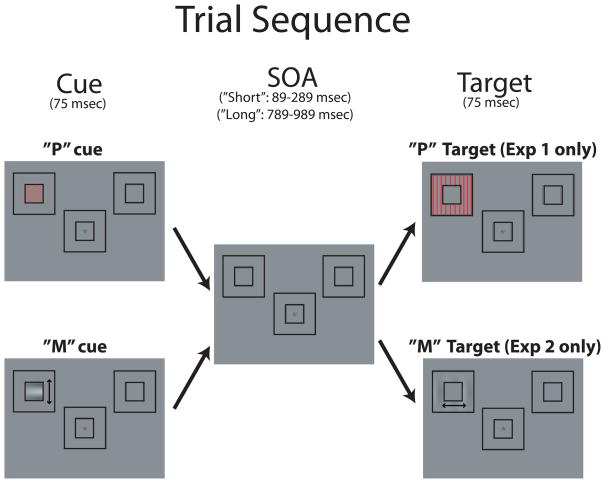
Participants performed an orientation discrimination task on a peripherally located target stimulus (Figure 1). The background screen consisted of a central fixation cross and two outline boxes (3.8°×3.8°) located 6° from fixation in the upper left and upper right visual fields. There were two cue types in this experiment, each measuring 1.4°×1.4° and occurring within the ever-present peripheral outline boxes. One cue type, designed to stimulate the P-pathway, was a red grating (9c/d) rotated 45° that was isoluminant with the background. The other cue-type, referred to as the M-Cue, was designed to stimulate the M-pathway and thus had low spatial frequency (2c/d), low luminance contrast (using Michelson contrast = 8% from values obtained in CIELAB color space; Wyszecki & Stiles, 1982), and motion (1.5 cycles for 75ms). The cue was perceived to move up or down with equal probability. The target in this experiment (a “P target”) had the same properties as the P-cue except that the target was a larger outline box (3.8°×3.8°), and the grating was oriented either horizontally or vertically. Participants were instructed to press one button with the right index finger if the target grating was horizontal or an adjacent button with their right middle finger if the grating was vertical.
Figure 1.
Trial sequence used in Experiments 1 and 2. The “P” cue (intended to produce strong activity in the parvocellular visual pathway) was a high spatial frequency grating that was isoluminant with the background. The “M” cue (intended to produce strong activity in the magnocellular pathway) was a low spatial frequency, low contrast, motion stimulus. Experiment 1 used a P-pathway target, whereas Experiment 2 used an M-pathway target. Both experiments used “P” and “M” cues. The trials depicted here are cued-location target trials (i.e., the cue and target appeared in the same spatial location). The arrows next to the “M” cue and “M” target are for illustrative purposes only and indicate the stimulus moved either up/down for the cue or right/left for the target. They were not seen during the experiment.
The cue was presented for 75 msec in the middle of one of the placeholder objects, randomly to the left or right location. The SOA between the cue and target was either short (89–289ms) or long (789–989ms). The short SOA was used in order to measure the initial capture of attention; the long SOA was used in order to assess how long the neural effects of capture would last as well as if IOR would occur with these stimuli. The cue location was not predictive of target location, and targets appeared (75 msec duration) in each location with the same probability. In order to prevent anticipatory responses triggered by the cue, a portion of trials (10%) were ‘catch’ trials, in which a cue appeared but with no subsequent target. Catch trials had an inter-trial-interval (ITI) of 1712–2212ms (cue offset to following cue onset). On trials where a target appeared, the ITI (target offset to following cue onset) was 1200–1500ms.
Isoluminance was determined individually for each subject using the minimally-distinct border method (MDB; Boynton and Kaiser, 1968). This method requires participants to adjust the luminance value of one of two juxtaposed stimuli so that the apparent border between the two stimuli is minimal. In the current studies, participants adjusted the red luminance value so that it matched the luminance of, or created a minimally distinct border when compared to, the gray background color stimulus. Each subject’s perceived isoluminance value was used for the cues and targets in their experimental session.
2. 3. EEG Recording
The electroencephalogram (EEG) was recorded from 96 electrode sites, referenced to the right mastoid, amplified at a bandpass of .01–100Hz and digitized at 250Hz. Electrodes located beneath and lateral to the outer canthus of each eye recorded the electro-oculogram. Trials containing eye movements, blinks, or excessive muscle activity were rejected off-line and not included in the analysis. EEG data were re-referenced offline to the average of the left and right mastoid. EEG was averaged by experimental condition to create ERP waveforms. The ERPs were low-pass filtered (Hz cutoff=100) to remove high-frequency noise and high-pass filtered (Hz cutoff=.01) with a single-pole causal filter to reduce low frequency drifts. Due to the close temporal proximity of the cue and target, the adjacent response (ADJAR) filter technique (Woldorff, 1993) was used to remove potential overlap, as has been implemented in previous visual and auditory attention studies using short SOAs (e.g., Fu et al., 2005; Hopfinger and Mangun, 1998, 2001; Hopfinger and Ries, 2005; Hopfinger & West, 2006; McDonald & Ward, 2000; McDonald, Teder-Sälejärvi, Di Russo & Hillyard, 2003; Talsma et al., 2005). Only trials with correct target responses between 200 and 1000 msec were included in the analysis.
3. Results
3. 1. Behavior
Reaction times (RT) to targets were analyzed using a 2×2×2 repeated measures ANOVA with the factors of Cue Validity (cued-location target vs. uncued-location target), SOA (short vs. long), and Congruency (congruent=P-cue, P-Target vs. incongruent=M-Cue, P-Target). The only significant main effect was for SOA (F(1,15) = 12.15, p = .003) with short SOA targets (mean=521ms) being faster than long SOA targets (mean=534ms). Significant Cue Validity × SOA, (F(1,15) = 22.3, p < .001) and Cue Validity × Congruency (F(1,15) = 14.7, p = .002) interactions were found. Given the significant interaction between validity and SOA, and our a priori interests in the effects at each SOA separately, we performed two additional ANOVAs: one for short SOA and another for long SOA, with the same factors as above except for SOA. For the short SOA trials, there was a significant main effect of Validity (F(1,15)= 16.38, p=.001) and an interaction between Validity and Congruency (F(1,15)=5.04 p =0.04). Cued-location targets were responded to significantly faster then uncued-location targets, and this difference was larger for congruent targets than incongruent targets (Table 1 shows means for all conditions). No other effects were significant at the short SOA. At the long SOA, the only significant effect was Congruency (F(1,15)=5.78, p =.032), with incongruent targets being responded to faster than congruent targets. 1
Table 1.
Behavioral measures for Experiment 1
| Experiment 1 (“P” targets) | |||
|---|---|---|---|
| REACTION TIME (msec) | |||
| Congruency (Cue, Target) | ISI | Cued | Uncued |
| Congruent (P,P) | Short | 515.2 (48.7) | 537.2 (51.8) |
| Incongruent (M,P) | Short | 519.7 (43.7) | 528.9 (50.1 |
| Congruent (P,P) | Long | 543.2 (51.8) | 539.5 (53.9) |
| Incongruent (M,P) | Long | 538.9 (48.3) | 528.9 (48.8) |
| ERRORS (%) | |||
|---|---|---|---|
| Congruency (Cue, Target) | ISI | Cued | Uncued |
| Congruent (P,P) | Short | 5.3 | 7.0 |
| Incongruent (M,P) | Short | 3.9 | 6.6 |
| Congruent (P,P) | Long | 4.8 | 5.6 |
| Incongruent (M,P) | Long | 4.3 | 4.5 |
Accuracy (errors in discrimination) was analyzed using the same factors used for RT. A significant main effect of cue validity was obtained F(1,15) = 20.92, p < .001, with cued targets (mean = 4.6%) resulting in fewer errors than uncued targets (mean = 6%). There was also a main effect for congruency F(1,15) = 6.1, p =.026, with incongruent targets (mean = 4.8%) showing fewer errors than congruent targets (mean = 6%). No other main effects or interactions were significant. Separate ANOVAs for each SOA revealed no significant effects at the long SOA, and only a significant effect of validity (F(1,15)=11.6, p =.004) at the short SOA.
3.2. Event-related Potentials
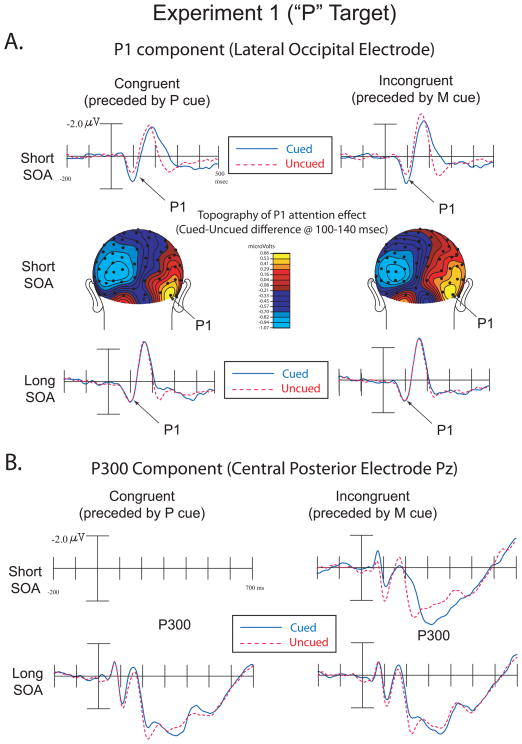
The amplitude of the target-evoked P1 component (80–120ms) was analyzed over electrode sites contralateral from target presentation using a repeated-measures ANOVA with the factors Cue Validity (cued-location vs. uncued-location), SOA (short vs. long), Cue Congruency (congruent=P cue vs. incongruent=M Cue), and Electrode (medial vs. lateral contralateral occipital-temporal electrodes). The electrodes correspond to the sites analyzed in previous reflexive attention studies (e.g. Hopfinger and Mangun, 1998; 2001; Hopfinger and Ries, 2005): medial occipital PO7 and PO8 and lateral occipital P7 and P8 according to the 10–10 system of electrode placement (Chatrian, Lettich and Nelson, 1985; Oostenveld and Praamstra, 2001). The analysis showed a main effect for Cue Validity (cued = 1.4μv, uncued = 1.29μv, F(1,15) = 6.69, p = .02), a Cue Validity by Electrode interaction (F(1,15) = 9.21, p = .008), and a three way interaction between Cue Validity, SOA, and Electrode (F(1,15) = 4.69, p = .047). No other main effects or interactions were significant at p< .05. Critically, the interaction between Cue Validity and Congruency did not approach significance (F(1,15) =.10, p = .75), providing evidence that the attentional capture effects on this level of processing were similar across the cue types. As with the behavioral analyses, we conducted separate ANOVAs for the short and long SOAs These analyses revealed that the 3-way interaction above was driven by the P1 component being larger for the cued than the uncued target, but only at the short SOA, and with larger effects over the lateral (versus more medial) occipital electrode (Figure 2a). The ANOVA for only short SOA trials revealed a significant effect of Validity (F(1,15)=6.6, p =.022). Critically, however, the interaction between Validity and Congruency did not approach significance (F(1,15)=.007, p =.936), as cued targets produced a larger P1 than uncued targets in both congruent (Congruent Cued=1.53 μv, Congruent Uncued = 1.18 μv) and incongruent conditions (Incongruent Cued = 1.65 μv, Incongruent Uncued = 1.33 μv). There was also a main effect of Electrode (PO7/PO8 = 1.1, P7/P8= 1.6, F(1,15)=6.7, p =.021), a Validity by Electrode interaction (F(1,15)=6.16, p =.025), and a Congruency by Electrode interaction (F(1,15)=18.43, p =.001). In the ANOVA for the long SOA condition, the only significant effects were a main effect of Electrode (PO7/PO8 = .97 μv, P7/P8=1.5 μv, F(1,15)=6.69, p =.021), and a Validity by Electrode interaction (F(1,15)=6.33, p =.024). No other main effects or interactions were significant (all p >.1).
Figure 2.
Experiment 1 (“P” Targets). A. ERPs showing the P1 component over lateral occipital electrodes in congruent and incongruent trials over short and long SOAs (top and bottom illustrations). These data are collapsed over contralateral scalp sites (i.e. left hemisphere data for right visual field targets were combined with right hemisphere data for left visual field targets). Topographic voltage maps from cued-uncued difference waves at the short SOA represent the distribution of neural activity highlighting the P1 attention effect (middle illustration). The right side of the topographic voltage maps represents neural activity contralateral to visual stimulation while the left side represents ipsilateral activity.
B. ERPs showing the P300 component in congruent and incongruent trials over short and long SOAs.
The amplitude of the target-evoked P300 (Figure 2b) component was analyzed with the same factors as above, but over the latency range 300–500 msec and over the central-posterior midline sites where this component is maximal (electrode factor was anterior vs. posterior midline electrodes, Cz and Pz respectively in the 10/10 system). This analysis revealed a main effect for Cue Validity (F(1,15) = 51.77, p < .001; Cued = 2.4μv, Uncued = 1.9μv), a main effect for SOA (F(1,15) = 16.4, p = .001; Short = 1.9μv, Long = 2.4μv), and a main effect for Congruency (F(1,15) = 16.26, p < .001; Congruent = 2.01μv, Incongruent = 2.31μv). Critically, the interaction between Validity and Congruency was not significant (F(1,15) = 0.31, p =.588). Two-way interactions were obtained for Cue Validity and SOA (F(1,15) = 30.08, p < .001), SOA and Congruency (F(1,15) = 15.15, p = .001), Cue Validity and Electrode (F(1,15) = 6.25, p = .024), and SOA and Electrode (F(1,15) = 19.05, p < .001). No other significant main effects or interactions were found. In the ANOVA for only the short SOA trials, there were significant main effects of Validity (F(1,15)=67.8, p <.001) and Congruency (F(1,15)=29.6, p <.001). Critically, and similar to the P1, the interaction of Validity and Congruency did not approach significance (F(1,15)=.025, p=.875). The only other significant effect was the Congruency by Electrode interaction (F(1,15)=4.66, p=.047). For the ANOVA on only the long SOA trials, a main effect of Electrode was obtained (F(1,15)=6.17, p =.025) and a Validity by Electrode interaction (F(1,15)=4.44, p =.05). There were no other significant main effects or interactions (all p >.2) at the long SOA.
While not of interest a priori, visual analysis of the ERP waveforms at Pz revealed an earlier difference between cued and uncued targets occurring primarily in the short SOA condition. Given that the cued-location targets produced a more negative going waveform, this effect may potentially be related to the negative difference (Nd) effect observed in previous studies (McDonald et al., 2003; McDonald and Ward, 2000). 2 This difference was analyzed with the same factors as above over electrode Pz (50–150ms). The analyses revealed a main effect for Validity (F(1,15)=5.82, p =.029; Cued = 0.12 μv, Uncued = 0.41 μv) and a main effect for Congruency (F(1,15)=11.01, p =.005; Congruent = 0.11 μv, Incongruent = 0.43 μv). The Validity by Congruency interaction, however, was not significant (F(1,15)=0.12, p =.734). No other main effects or interactions were significant, all p > .05. In the ANOVA for only the short SOA trials, there were significant main effects of Validity (F(1,15) = 7.36, p = .016; Cued = .008μv, Uncued = .427 μv) and Congruency (F(1,15)=11.37, p =.004; Congruent= −.035 μv, Incongruent = .470 μv). The Validity by Congruency interaction was, again, not significant (F(1,15)=0.023, p =.882). For the long SOA ANOVA there were no significant main effects or interactions (all p > .2). The interpretation of this cuing effect is discussed below.
4. Discussion
Based largely on behavioral studies, there is debate regarding whether either the M or P visual pathway is unique or critical in triggering attentional capture. Previous ERP studies of attentional capture have revealed that multiple levels of processing are affected by non-predictive peripheral cues, but these studies used cue stimuli that likely stimulated both the M and P visual pathways. In the present study, we sought to isolate the effects of capture within each pathway to investigate whether the effects on neural processing are similar or different. The behavioral results from the present study do not provide unequivocal support for either pathway alone being responsible for triggering a reflexive shift of attention. Specifically, the cue validity by SOA interaction was significant in the RT data (for short SOA trials) showing that overall RTs were faster to cued relative to uncued targets. However, there was also a validity by congruency interaction at this short SOA. This was driven by the validity effect being stronger in the congruent condition versus the incongruent condition. Although this interaction suggests that the effects are not identical in each congruency condition, there was a similar trend in both conditions for RTs to be faster for the cued-location targets than the uncued-location targets. Given the somewhat ambiguous behavioral results, the ERP measures can provide additional data critical for determining if these pathways operate via similar mechanisms at stages of processing preceding overt behavior. Whereas behavioral measures can result from a nonlinear interaction of many stages of processing, ERPs provide a more direct and isolated measure of specific stages of processing.
The present ERP results provide new evidence that the M and P pathways can trigger reflexive mechanisms that act on the same stages of target processing. This was evidenced as increased P1 and P300 amplitudes for cued relative to uncued-location targets, at short SOAs. Critically, at the short SOA, there was no interaction between validity and congruency, as the benefit for cued-location targets over uncued-location targets was the same in both the congruent and incongruent conditions. Thus, these data provide novel evidence that involuntary attention mechanisms that modulate neural processing as early as ~ 100 msec can be triggered by either the M or P visual pathway. Furthermore, the cuing validity effects triggered by both of these pathways were fast-acting but transient, being robust at short cue-to-target SOAs and absent at long SOAs.
Although not expected, the current data also revealed another, even earlier effect of cuing validity at the short SOA, as cued-location targets showed a negative shift in the ERP over central parietal regions relative to uncued-location targets. This effect obtained in the present study provides further evidence that each pathway can trigger a reflexive shift of attention. This negative shift may relate to the negative difference wave observed in previous cuing studies, referred to as an “Nd” (“Negative difference”; e.g., McDonald and Ward, 2000; McDonald et al., 2003). An Nd here would be generally consistent with prior research showing that spatially non-predictive auditory cues capture attention and enhance subsequent visual target activity at short cue to target intervals (McDonald et al., 2003). However, it is unclear if the present negative difference effect should be directly compared to the Nd seen in previous studies. Critically, in previous studies (McDonald and Ward, 2000; McDonald et al., 2003) the Nd occurred after the P1 component, whereas in the present study, this negativity begins before the P1 and about 50msec earlier than the earliest Nd reported in any previous reports that we are aware of. This effect will be discussed more in the general discussion section below.
Although this experiment provided new evidence that P and M-cues have similar effects on attentional capture, it is possible that the capture of attention by P Cues may not have been solely bottom-up in this experiment. Specifically, since the targets were always known to be P-pathway targets, top-down control setting (e.g. expecting a P-target) may have influenced the effects (i.e., caused the capture by the matching P-cues). In other words, the present results could be accounted for by postulating that whereas the M pathway triggers a completely automatic series of effects, the P pathway produces these effects only in the case when the cue matches a salient feature of the expected target. A related interpretation is that the effects of the P-cues were dependant on within-pathway priming of the P-pathway. Experiment 2 was designed to test if the behavioral and neural effects of attentional capture observed in Experiment 1 remain the same when the target does not stimulate the P-pathway and instead stimulates the M-pathway.
5. Methods. Experiment 2: M-pathway Targets
5.1. Participants
Participants consisted of 16 healthy, right-handed adults (average age 27.9 years; 7 females) with 20/20 or corrected-to-20/20 color vision. Each participant gave informed consent, as approved by the IRB at the University of North Carolina at Chapel Hill, and was reimbursed $10 per hour for their time.
5.2. Procedure
The target in this experiment was designed to stimulate the M-pathway. The timing and all other stimuli were identical to that used in Experiment 1. The target in Experiment 2 (M target) was the same size as in Experiment 1, but it was a 1c/d Gabor grating with the same luminance contrast as the M cue, and this target grating had apparent motion to the left or right (1.5 cycles for 75ms). Participants were instructed to press one button with the right index finger if motion was to the left and a different button with their right middle finger if motion was to the right. EEG recording and analyses were the same as Experiment 1, and isoluminance was determined for each participant as in Experiment 1.
6. Results (Experiment 2)
6.1. Behavior
RTs to targets were analyzed using a 2×2×2 repeated measures ANOVA with the main factors of cue validity (cued or uncued), SOA (short or long) and Congruency (congruent or incongruent). There were significant main effects of Cue Validity (F(1,15) = 12.37, p =.003; Cued = 431.8, Uncued = 443.5), SOA (F(1,15) = 6.49, p = .022; Short = 432.6, Long = 442.8), and Congruency (F(1,15) = 10.97, p =.005; Congruent = 434.7, Incongruent = 440.7). The Validity by Congruency interaction was not significant (F(1,15)=1.99, p =.179). The only significant interaction was a Cue Validity × SOA interaction (F(1,15) = 19.68, p < .001). No other interactions were significant, all p > .1. Separate ANOVAs for each SOA revealed that at the short SOA, there was a significant effect of Validity (F(1,15) = 21.21, p <.001) and a near significant effect of Congruency (F(1,15) =3.81, p = .069), but critically, no interaction between Validity and Congruency (F(1,15) =.22, p = .83). Cued targets were responded to faster than uncued targets, in both the congruent and incongruent conditions. (Table 2 contains condition means). In the ANOVA for the long SOA condition, there were no significant effects (all p>.05).
Table 2.
Behavioral measures for Experiment 2
| Experiment 2 (“M” targets) | |||
|---|---|---|---|
| REACTION TIME (msec) | |||
| Congruency (Cue, Target) | ISI | Cued | Uncued |
| Congruent (M,M) | Short | 417.6 (57.9) | 439.7 (76.5) |
| Incongruent (P,M) | Short | 425.1 (58.7) | 447.8 (72.8) |
| Congruent (M,M) | Long | 437.8 (60.9) | 443.5 (65.7) |
| Incongruent (P,M) | Long | 446.7 (59.8) | 443.1 (63.2) |
| ERROR (%) | |||
|---|---|---|---|
| Congruency (Cue, Target) | ISI | Cued | Uncued |
| Congruent (M,M) | Short | 3.4 | 8.6 |
| Incongruent (P,M) | Short | 4 | 9.1 |
| Congruent (M,M) | Long | 3.4 | 4.4 |
| Incongruent (P,M) | Long | 3.6 | 6.6 |
Target errors were analyzed using the same model described above for RTs. Significant main effects were found for Validity (F(1,15) = 8.84, p =.009; Cued =3.6%, Uncued =8%) and SOA (F(1,15) = 6.55, p =.022, Long SOA =4.4%; Short SOA = 6.3%). The only significant interaction was Cue Validity × SOA (F(1,15) = 8.3, p =.011). Separate ANOVAs for the long and short SOA revealed that for the short SOA, the only significant effect was the main effect of Validity (F(1,15) = 9.96, p = .007). For the long SOA, there was a significant main effect of Congruency ((F(1,15) = 5.97, p = .027), a near significant main effect of Validity (F(1,15) = 4.42, p = .053) and a near significant interaction between Validity and Congruency (F(1,15) = 4.32, p = .055).
6.2. Event-related Potentials
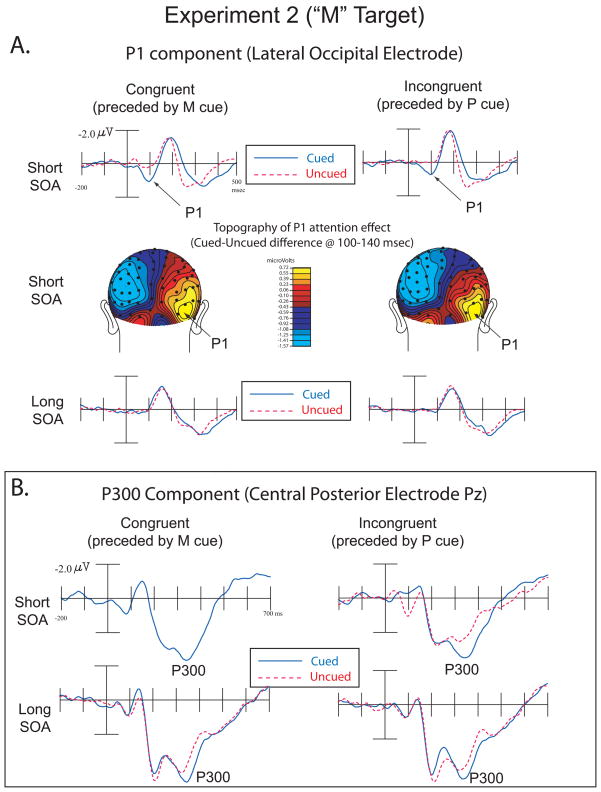
The amplitude of the target-evoked contralateral P1 component (75–115ms) was analyzed using a repeated-measures ANOVA with the factors Cue Validity (cued or uncued), SOA (short or long), Congruency (congruent or incongruent), and Electrode (medial P07/P08 or lateral P7/P8). The analysis showed main effects of Cue Validity (F(1,15) = 12.25, p = .003; Cued = .42μv, Uncued = .08μv), SOA (F(1,15) = 4.85, p = .043 (Short = .56μv, Long = .07μv), and Congruency (F(1,15) = 6.23, p = .025 (Congruent = .35μv, Incongruent = .14μv). Significant two-way interactions were obtained between Cue Validity and SOA (F(1,15) = 13.78, p = .002), SOA and Congruency (F(1,15) = 7.37, p = .02), and Congruency and Electrode (F(1,15) = 20.14, p < .001). A three-way interaction between SOA, Congruency and Electrode was also found (F(1,15) = 10.66, p = .005). No other main effects or interactions were significant (all p > .05). We again performed two separate ANOVAs, without the factor of SOA, for each SOA separately. For the short SOA, there was a significant main effect of Validity (F(1,15) = 16.66, p = .001) and a significant effect of Congruency (F(1,15) = 8.8, p = .01). Critically, however, the interaction between Validity and Congruency did not approach significance (F(1,15) = .318, p = .581), as cued-location targets produced larger P1s than uncued-location targets in both congruent (Congruent Cued =1.11 μv; Congruent Uncued =.44 μv) and incongruent conditions (Incongruent Cued =.64 μv; Incongruent Uncued.05 μv). There was also a Congruency by Electrode interaction (F(1,15)=22.6, p<.001) at the short SOA. In the ANOVA for the long SOA, there were no significant effects or interactions (all p > .1).
The amplitude of the target-evoked P300 component (300–500ms) was analyzed over central-midline/posterior-midline electrode sites Cz and Pz respectively using a repeated-measures ANOVA with the factors Cue Validity (cued or uncued), SOA (short or long), Congruency (congruent or incongruent), and Electrode (central versus more posterior). This analysis revealed a main effect for Cue Validity (F(1,15) = 36.19, p < .001; Cued = 2.59μv, Uncued = 1.89μv), and a main effect for SOA (F(1,15) = 32.92, p < .001; Short = 1.79μv, Long = 2.69μv). A two-way interaction was obtained for SOA and Congruency (F(1,15) = 13.16, p = .003; Short Congruent =1.59μv, Short Incongruent=1.97μv, Long Congruent=2.83μv, Long Incongruent =2.56μv), and for SOA and Electrode (F(1,15) = 20.22, p < .001). No other significant main effects or interactions were found, p < .05. As with the P1 above, we performed 2 separate ANOVAs, without the factor of SOA, for each SOA separately. For the short SOA, there was a significant main effect of Validity (F(1,15) = 31.42, p < .001; Cued= 2.22μv, Uncued = 1.34μv) and a main effect of Congruency(F(1,15) = 12.47, p =.003; Congruent= 1.59μv, Incongruent= 2.02μv) (Figure 3B, top). Critically, the Validity by Congruence interaction was not significant (F(1,15) =.825, p = .378), as again the effect of validity did not depend on congruence between cue and target type. For the long SOA, there was a significant main effect of Validity (F(1,15) = 13.42, p =.002; Cued= 2.95μv, Uncued = 2.43μv) and a main effect of Congruency(F(1,15) = 7.39, p =.016; Congruent= 2.83μv, Incongruent= 2.56μv) (Figure 3B bottom). No other main effects or interactions were significant (all p > .05).
Figure 3.
Experiment 2 (“M” Targets). A. ERPs showing the P1 component over lateral occipital electrodes in congruent and incongruent trials over short and long SOAs (top and bottom illustrations). Topographic voltage maps from cued-uncued difference waves at the short SOA represent the distribution of neural activity highlighting the P1 attention effect (middle illustration).
B. ERPs showing the P300 component in congruent and incongruent trials over short and long SOAs.
As in experiment 1, there was a noticeable negative shift in the central electrodes at short latency. This negative difference at 50–150 msec was examined using the factors and electrode used in this analysis for Experiment 1. The analyses revealed a main effect for Validity (F(1,15)=11.81, p =.004; Cued = −.02 μv, Uncued = .34 μv) and a Validity by SOA interaction (F(1,15)=11.64, p =.004). The Validity by Congruency interaction was not significant (F(1,15)=.703, p =.415). No other main effects or interactions were significant (all p > .1). In the ANOVA for only the short SOA trials, the only significant main effect was for Validity (F(1,15) = 18.45, p = .001; Cued = −.178μv, Uncued = = .451 μv). The Validity by Congruency interaction was not significant (F(1,15)=.309, p =.587). For the long SOA ANOVA there were no significant main effects or interactions (all p > .05).
7. Discussion (Experiment 2)
The results from Experiment 1 showed that both M and P cues can capture attention and bias visual processing when the target stimulates the P-pathway. In Experiment 2, we were able to show that those results were not restricted to target processing along the P-pathway, but also occur for M-pathway targets. Specifically, behavioral performance was better for cued-location targets compared to uncued-location targets, and this did not interact with congruency between cue and target type. The P1 component was also enhanced by cuing validity, being significantly larger for cued-location targets relative to uncued-location targets. Critically, these cuing effects occurred for both congruent and incongruent conditions at short SOAs, as was the case in Experiment 1. This provides further evidence that the effects of attentional capture at this early level of processing is not contingent on top-down expectation and attentional control settings, nor on priming within one visual pathway. As in Experiment 1, the long SOA condition did not show any significant effect of cuing on P1 amplitude, suggesting the attention effect on this component is quite transient. At later stages of target processing, we found that the target-evoked P300 was larger for cued-location targets compared to uncued-location targets in both congruency conditions and at both SOAs. We expected this finding at the short SOA based on previous literature, but we believe this is the first report of a P300 attention effect for reflexive attention at long SOAs. This finding thus provides preliminary evidence that target processing at this stage may be affected somewhat differently in the M versus P pathway, since a P300 effect occurred at the long SOA only in this experiment, where the target was known to be an M-pathway target.
8. General Discussion
There is currently disagreement about whether attentional capture is predominately triggered by the magnocellular visual system or if parvocellular activation can also lead to attentional capture. The present experiments provide new evidence that both the magnocellular and parvocellular visual pathways can trigger a reflexive shift of attention and enhance both early perceptual level processing and later higher-order processing stages. This was evidence by faster RTs and enhanced P1 and P300 ERP components to cued-location targets, relative to uncued-location targets at short cue to target intervals. These effects did not depend on the type of target (i.e., P-target or M-target), or the congruence between cue type and target type. Thus, these data provide new evidence that these neural effects of reflexive attentional capture do not depend on within-pathway priming or top-down expectations of target type, and that both the M and P visual pathway can trigger these neural effects.
One possible concern with the present results is whether the current lack of validity by congruency interactions could be due to the P and M cues not being strong enough to produce differential effects. Indeed, Zhang & Luck (2009) recently found that feature based attention could produce effects as early as space-based attention, suggesting that attending to motion (M pathway) or attending to color (P pathway) should produce selective effects unique to the attended attribute. However, a possible critical difference in our study is that our targets were presented in isolation, whereas Zhang and Luck (2009) showed that the early effects of feature attention were dependant on the presence of simultaneously-presented distractors. In their study, the early selective effects were only obtained when a distractor was present. Theoretically, the lack of validity by congruency interactions in the present study could occur if there were ceiling (or floor) effects. This explanation could be applicable to accuracy measures, since subjects were almost perfect in all conditions. However, the RTs show an interaction between validity and congruency, providing evidence that attentional capture had differential effects on overt behavior, depending on the visual processing stream activated by the cue, and that the present design was sensitive enough to detect this interaction. In addition, the P1 and P3 effects are robust in all conditions at the short SOA, and similar in size to that seen in previous studies, even in the incongruent conditions. Thus, the lack of interactions does not seem to be due to insensitive design issues..
It is noteworthy that previous crossmodal attention studies have found that auditory and tactile cues can produce attention effects on visual target processing as early as the P1 effects found in the present study (McDonald et al., 2005; Eimer and Driver, 2000). Therefore, it may seem unsurprising that the present unimodal visual study would find effects for two different varieties of visual cue stimuli. However, based on results from behavioral research, this is not an obvious conclusion to draw, because previous studies had concluded that only one of these visual pathways was responsible for attentional capture. Furthermore, it is possible that some auditory stimuli could trigger similar attentional capture as visual stimuli stimulating a certain pathway, even when other varieties of visual stimuli (stimulating others pathways) could not.
The stimuli manipulations in the current study were designed to primarily target either the M or P pathway. It is unclear what, if any, effect the koniocellular (K) visual pathway may have had in the present experiments. The K stream, like the M stream, has a fast conduction speed, which would also likely show faster sensory processing compared to P targeting stimuli. Future research with stimuli designed to specifically target the K- stream (e.g., blue-on cells) would be needed to assess whether this pathway makes any unique contributions to the capture of attention (Callaway, 2005). Regardless of the possible contributions of K-pathway, the present data provide new behavioral and neural evidence that the parvocellular pathway can trigger attentional capture in a highly similar manner as does the magnocellular system.
It has been noted that the Nd effect in crossmodal attention studies may resemble an involuntary shift of attention that first modulates neural activity in a multimodal brain region prior to the cortical region responsible for processing the primary target properties (McDonald and Ward, 2000; McDonald, et al., 2003). Our results are consistent with this hypothesis; however, there is a need for further research on this component regarding its role in unimodal attentional capture, for it has been most often reported for studies of multimodal attention (e.g. Kennett et al., 2001; McDonald et al., 2001). As mentioned earlier, the current negativity effect may not be the same Nd as observed in those studies, as suggested by the very early onset of the present effects. Further research is needed to replicate the current early latency effects in unimodal visual attention studies and to directly compare it to the crossmodal Nd effects previously observed.
While the functional processing characteristics of the M and P pathways are different, activation of these systems appears to trigger reflexive attention and bias subsequent neural processing in highly similar ways. The results of the current experiments suggest attentional capture is not dependent on the activation of one visual processing pathway more than the other. The present study provides new behavioral and electrophysiological evidence of attentional capture through both the magnocellular and parvocellular visual pathways.
Highlights.
We show that magnocellular and parvocellular systems trigger attentional capture.
This capture enhances both early sensory and later high-order neural processing.
These enhancements are transient, occurring only at short cue to target interval
These effects are not dependent on top-down control settings.
These effects are not dependant on within-pathway priming.
Acknowledgments
This research was supported by grant MH066034 from the National Institute of Mental Health (PI: Joseph B. Hopfinger).
Footnotes
Faster RTs to short SOA targets compared to long SOA targets is in the opposite direction of some other studies. Although it is unclear what may cause this difference, it may be at least partially due to the inclusion of catch trials here and the relatively large difference between short and long SOA intervals, as a similar pattern has been observed in a previous similar study (Hopfinger & Mangun, 1998). Specifically, on the longer of the long SOA trials, subjects’ alertness may drop off considerably as they mistakenly assume a target is not going to occur on that trial (catch trials could be shorter than some long SOA target trials). Since the main focus of the present study is on the short SOA, however, this possible strategy should not affect the main, and most critical, results discussed here.
We thank an anonymous reviewer for pointing this out.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams RA, Christ SE. Motion onset captures attention. Psychological Science. 2003;14:427–432. doi: 10.1111/1467-9280.01458. [DOI] [PubMed] [Google Scholar]
- Atchley P, Kramer AF, Hillstrom AP. Contingent capture for onsets and offsets: Attentional set for perceptual transients. Journal of Experimental Psychology: Human Perception & Performance. 2000;26:594–606. doi: 10.1037//0096-1523.26.2.594. [DOI] [PubMed] [Google Scholar]
- Boynton RM, Kaiser PK. Vision: the additivity law made to work for heterochromatic photometry with bipartite fields. Science. 1968;161:366–368. doi: 10.1126/science.161.3839.366. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. Journal of Physiology. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. American Journal of EEG technology. 1985;25:83–92. [Google Scholar]
- Cheng A, Eysel UT, Vidyasagar TR. The role of the magnocellular pathway in serial deployment of visual attention. European Journal of Neuroscience. 2004;20:2188–2192. doi: 10.1111/j.1460-9568.2004.03675.x. [DOI] [PubMed] [Google Scholar]
- Cole G, Kentridge R, Heywood C. Object onset and parvocellular guidance of attentional allocation. Psychological Science. 2005;16:270–274. doi: 10.1111/j.0956-7976.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annual Review of Psychology. 1997;48:269– 297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Eimer M, Driver J. An event-related brain potential study of cross-modal links in spatial attention between vision and touch. Psychophysiology. 2000;37:697–705. [PubMed] [Google Scholar]
- Folk C, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Folk CL, Remington RW, Wright JH. The structure of attentional control: Contingent attentional capture by apparent motion, abrupt onset, and color. Journal of Experimental Psychology: Human Perception & Performance. 1994;20:317–329. doi: 10.1037//0096-1523.20.2.317. [DOI] [PubMed] [Google Scholar]
- Fu S, Fan S, Chen L, Zhao Y. The attentional effects of peripheral cueing as revealed by two event-related potential studies. Clinical Neurophysiology. 2001;112:172– 185. doi: 10.1016/s1388-2457(00)00500-9. [DOI] [PubMed] [Google Scholar]
- Fu S, Greenwood PM, Parasuraman R. Brain mechanisms of involuntary visuospatial attention: An event-related potential study. Human Brain Mapping. 2005;25:378–390. doi: 10.1002/hbm.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo RM, Abrams RA, Moscovitch M, Pratt J. Isoluminant motion onset captures attention. Attention, Perception, & Psychophysics. 2010;72:1311–1316. doi: 10.3758/APP.72.5.1311. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Mangun GR. Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychological Science. 1998;9:441–447. doi: 10.1111/1467-9280.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Mangun GR. Tracking the influence of reflexive attention on sensory and cognitive processing. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:56–65. doi: 10.3758/cabn.1.1.56. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Ries AJ. Automatic versus contingent mechanisms of sensory-driven neural biasing and reflexive attention. Journal of Cognitive Neuroscience. 2005;17:1341–1352. doi: 10.1162/0898929055002445. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, West VM. Interactions between endogenous and exogenous attention on cortical visual processing. NeuroImage. 2006;31:774–789. doi: 10.1016/j.neuroimage.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Jasper H. The ten twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. In: Long JB, Baddeley AD, editors. Attention & performance IX. Hillsdale, NJ: Erlbaum; 1981. pp. 187–203. [Google Scholar]
- Kaplan E. The M, P, and K pathways of the primate visual system. In: Chaupa L, Werner J, editors. Visual Neurosciences. MIT; 2004. pp. 481–493. [Google Scholar]
- Kennet S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous Spatial attention under different postures: Convergent evidence from psychphysics and ERPs. Journal of Cognitive Neuroscience. 2001;13:462–478. doi: 10.1162/08989290152001899. [DOI] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA. Exogenous attentional selection of transparent superimposed surfaces modulates early event-related potentials. Vision Research. 2005;45:3004–3014. doi: 10.1016/j.visres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Kulikowski JJ, Robson AG, Murray IJ. Scalp VEPs and intra-cortical responses to chromatic and achromatic stimuli in primates. Documenta Ophthalmologica. 2002;105:243–279. doi: 10.1023/a:1020557105243. [DOI] [PubMed] [Google Scholar]
- Lu S. Cue duration and parvocellular guidance of visual attention. Psychological Science. 2006;17:101–102. doi: 10.1111/j.1467-9280.2005.01671.x. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Salejarvi WA, Di Russo F, Hillyard SA. Neural basis of auditory-induced shift in visual time-order perception. Nature Neuroscience. 2005;8:1197–1202. doi: 10.1038/nn1512. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Salejarvi WA, Di Russo F, Hillyard SA. Neural substrates of perceptual enhancement by cross-modal spatial attention. Journal of Cognitive Neuroscience. 2003;15:10–19. doi: 10.1162/089892903321107783. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Teder-Salejarvi WA, Heraldez D, Hillyard SA. Electrophysiological evidence for the “missing link” in crossmodal attention. Canadian Journal of Experimental Psychology. 2001;55:141–149. doi: 10.1037/h0087361. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Ward LM. Involuntary listening aids seeing: Evidence from human electrophysiology. Psychological Science. 2000;11:167–171. doi: 10.1111/1467-9280.00233. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Ward LM, Kiehl KA. An event-related brain potential study of inhibition of return. Perception & Psychophysics. 1999;61:1411–1423. doi: 10.3758/bf03206190. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Praamstra P. The five percent electrode system for high-resolution EEG and ERP measurements. Clinical Neurophysiology. 2001;112:713–719. doi: 10.1016/s1388-2457(00)00527-7. [DOI] [PubMed] [Google Scholar]
- Pitzalis S, Di Russo F, Spinelli D. Loss of visual information in neglect: the effect of chromatic–versus luminance-contrast stimuli in a “what” task. Experimental Brain Research. 2005;163:527–534. doi: 10.1007/s00221-004-2207-4. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attention and Performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Prime DJ, Ward LM. Inhibition of return from stimulus to response. Psychological Science. 2004;15:272–276. doi: 10.1111/j.0956-7976.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- Ruchkin D, Johnson R, Canoune H, Ritter W, Hammer M. Multiple sources of P3b associated with different types of information. Psychophysiology. 1990;27:157–176. doi: 10.1111/j.1469-8986.1990.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Snowden RJ. Visual attention to color: Parvocellular guidance of attentional resources? Psychological Science. 2002;13:180–184. doi: 10.1111/1467-9280.00433. [DOI] [PubMed] [Google Scholar]
- Steinman B, Steinman S, Lehmkuhle S. Transient visual attention is dominated by the magnocellular stream. Vision Research. 1997;37:17–23. doi: 10.1016/s0042-6989(96)00151-4. [DOI] [PubMed] [Google Scholar]
- Stormer VS, McDonald JJ, Hillyard SA. Cross-modal cueing of attention alters appearance and early cortical processing of visual stimuli. Proceedings of the National Academy of Sciences. 2009:1–6. doi: 10.1073/pnas.0907573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Abrupt luminance change pops out; abrupt color change does not. Perception & Psychophysics. 1995;57:637–644. doi: 10.3758/bf03213269. [DOI] [PubMed] [Google Scholar]
- Talsma D, Slagter HA, Nieuwenhuis JH, Kok A. The orienting of visuospatial attention: An event-related brain potential study. Cognitive Brain Research. 2005;25:117–129. doi: 10.1016/j.cogbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- White BJ, Boehnke SE, Marino RA, Itti L, Munoz DP. Color-related signals in the primate superior colliculus. The Journal of Neuroscience. 2009;29:12159–12166. doi: 10.1523/JNEUROSCI.1986-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken, Kramer A, Vanesse L, Donchin E. The performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: Analysis and correction. Psychophysiology. 1993;30:98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Wyszecki G, Stiles WS. Color science: Concepts and methods, quantitative data and formulae. 2. New York: Wiley; 1982. [Google Scholar]
- Yantis S, Hillstrom AP. Stimulus-driven attentional capture: Evidence from equiluminant visual objects. Journal of Experimental Psychology: Human Perception & Performance. 1994;20:95–107. doi: 10.1037//0096-1523.20.1.95. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: Evidence from visual search. Journal of Experimental Psychology: Human Perception and Performance. 1984;5:625–638. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nature Neuroscience. 2009;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]