Abstract
Background and Objectives
We sought to determine parameters to guide the decision of retreatment in patients with Kawasaki disease (KD) who remained febrile after initial intravenous immunoglobulin (IVIG).
Subjects and Methods
A total of 129 children with KD were studied prospectively. Patients were treated with IVIG 2 to 9 days after the onset of disease. Laboratory measures, such as white blood cell (WBC), percentage of neutrophils, C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT-proBNP), were determined before and 48 to 72 hours after IVIG treatment. Patients were classified into IVIG-responsive and IVIG-resistant groups, based on the response to IVIG.
Results
Of a total of 129 patients, 107 patients (83%) completely responded to a single IVIG therapy and only 22 patients (17%) required retreatment: 14 had persistent fever and 8 had recrudescent fever. There was no significant difference between the groups in age, gender distribution, and duration of fever to IVIG initiation, but coronary artery lesions developed significantly more often in the resistant group than in the responsive group (31.8% vs. 2.8%, p=0.000). Compared with pre-IVIG data, post-IVIG levels of WBC, percentage of neutrophils, CRP, and NT-proBNP decreased to within the normal range in the responsive group, whereas they remained high in the resistant group. Multivariate logistic regression indicated that neutrophil counts, CRP, and NT-proBNP were independent parameters of retreatment.
Conclusion
Additional therapy at an early stage of the disease should be administered for febrile patients who have high values of CRP, NT-proBNP, and/or neutrophil counts after IVIG therapy.
Keywords: Kawasaki disease, Intravenous immunoglobulins, Retreatment
Introduction
Intravenous immunoglobulin (IVIG) infusion is an effective therapy for acute Kawasaki disease (KD).1) Administration within a few days of illness of a single dose of IVIG, in combination with high-dose aspirin, ceases fever, rapidly resolves clinical signs and symptoms of inflammation, and reduces the incidence of coronary artery aneurysms.1),2) However, approximately 10% to 20% of KD patients have persistent or recrudescent fever, despite IVIG therapy.2),3)
When fever persists or recurs with signs of mucocutaneous inflammation after initial IVIG therapy, retreatment can be indicated. However, when the patients have fever with few clinical signs of ongoing inflammation, these patients pose a therapeutic dilemma. The KD patients who fail to respond to initial IVIG treatment should be reassessed to exclude the pos-sibility of a bacterial infection. Although current diagnostic criteria for KD require exclusion of infectious diseases before making a definite diagnosis, there are actually several reports of patients with typical features of KD, including coronary artery aneurysm, who also have had a documented infection.4),5) In addition, we have sometimes experienced KD patients who became afebrile with IVIG treatment, but shortly febrile again due to newly developed viral infection, pharyngotonsillitis, gastroenteritis, or urinary tract infection during hospitalization. In such cases, the values of acute phase reactants were usually within the normal range and fever subsided without the use of IVIG. In patients who remain febrile despite IVIG therapy, our institutional protocol requires that we always deliberate on the values of acute phase reactants, when a need for additional therapy is recognized. If the patients become afebrile after treatment, no laboratory test is performed. Most physicians clinically judge post-IVIG fever to be at-tributable to active KD, rather than to other causes,2),6) and tend to retreat this group of patients in the absence of the systemic evaluation required to guide the decision of treatment. We believe that there is a need for objective laboratory measures that identify KD patients who remain febrile after IVIG treat-ment and therefore need additional therapy.
Our goal was to determine from routine laboratory meas-ures the parameters that may serve to guide retreatment in KD patients who remained febrile after initial IVIG therapy.
Subjects and Methods
During the study period between November 2008 and September 2010, a total of 129 children who met the diagnostic criteria for KD7) were prospectively enrolled. Subjects comprised 72 boys (55.8%) and 57 girls, aged 2 months to 11 years. All patients were treated with IVIG 2 g/kg for 2 to 9 days after the onset of disease, and were also given aspirin 80 to 100 mg/kg/day until defervescence, and then 5 mg/kg/day as a single daily dose. When the patients failed to defervesce after the first IVIG therapy, they were retreated with a second dose of IVIG (n=14). When they remained still febrile despite a second dose of IVIG therapy, then pulse methylprednisolone (30 mg/kg/day for 3 days) was administered (n=8).
We measured white blood cell (WBC), percentage of neutrophils in WBCs (% neutrophils), hematocrit, platelet counts, serum sodium, total bilirubin, aspartate aminotransferase, alanine aminotransferase (ALT), protein, albumin, C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT-proBNP), before and 48 to 72 hours after IVIG treatment. For patients who failed to respond to the second dose of IVIG, laboratory measures were repeated before pulse steroid therapy. Coronary arteries were assessed by echocardiography performed at the time of diagnosis and at discharge, and repeated at weeks 2, 4, and 8 after treatment, and then as needed thereafter. Coronary artery lesions (CALs) were diagnosed according to the criteria of the Japanese Ministry of Health and Welfare.8) Patients who presented with CAL before the initial treatment were excluded from the study.
Subjects were divided into 2 groups according to responsiveness to IVIG treatment: IVIG-responsive patients (n=107) and IVIG-resistant patients (n=22). Laboratory measures before and after IVIG therapy were compared in each group. We selected the variables that showed a different change after IVIG therapy between the groups. Pre- and post-IVIG concentrations of each variable between the groups were compared. The value of the fractional change (FC) of each variable between the groups was also compared, as described previously.9) FC was defined as follows:
FC=(Y-X)/X
In which X represents data before IVIG treatment and Y represents data at 48 to 72 hours after IVIG treatment.
This study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (#ECT 230-2-28) and written informed consents were obtained from the parents of each patient.
Definitions
Duration of fever was defined as the time from the onset of disease to the start of initial IVIG infusion. Persistent fever was defined as temperature ≥38℃ that persisted beyond 48 hours after completion of the IVIG infusion. Recrudescent fever was defined as temperature <37.5℃ for more than 24 hours after IVIG infusion, followed by temperature ≥38℃. IVIG-responsive patients were defined as patients who had defervescence within 48 hours after initial IVIG infusion. IVIG-resistant patients were defined as those who required additional rescue therapy, owing to either persistent or recrudescent fever at least 48 hours after the end of initial IVIG infusion.
Data analyses
All data were analyzed with the Statistical Package for the Social Sciences for Windows software (SPSS) version 12.0 (SPSS Inc, Chicago, IL, USA). Data are presented as either mean±SD for continuous variables or as the number (percen-tage) of patients for categorical variables. Intragroup variables were compared using the paired t-test, and inter-group variables and FCs using the Student t-test. Proportions were compared using either the χ2 test or the Fisher's exact test. To determine the cutoff value of each parameter found to be significant by FC, receiver operating characteristic (ROC) curves were used. Multivariate logistic regression analysis was used to determine independent parameters of retreatment and results were expressed as an odds ratio with a 95% confidence interval (CI). A 95% CI that did not include 1.0 was interpreted to indicate statistical significance. A level of p<0.05 was considered as statistically significant.
Results
Clinical characteristics of intravenous immunoglobulin-resistant patients
Of a total of 129 patients, 107 patients (83%) completely responded to a single IVIG therapy and 22 patients (17%) required retreatment: 14 had persistent fever and 8 had recrudescent fever. Of the 14 patients with persistent fever, 7 patients achieved defervescence after the second dose of IVIG. However, the remaining 7 failed to respond to the second IVIG therapy and received pulse methylprednisolone therapy. In contrast, 7 of the 8 patients with recrudescent fever achieved defervescence after the second course of IVIG. However, according to fever pattern, no significant difference in responsiveness to a second dose of IVIG was found (p=0.086).
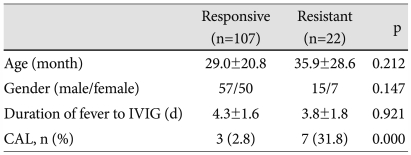
IVIG-resistant patients did not differ from the IVIG-responsive patients with respect to age, gender distribution, and duration of fever measured until IVIG initiation. However, CALs developed significantly more often in the IVIG-resistant patients than in IVIG-responsive patients (Table 1).
Table 1.
Comparison of basic characteristics between IVIG-responsive and IVIG-resistant patients
IVIG: intravenous immunoglobulin, CAL: coronary artery lesion
Changes in laboratory measures after intravenous immunoglobulin therapy in responsive patients
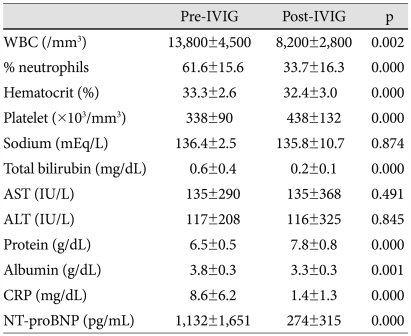
Compared with pre-IVIG data, post-IVIG levels of WBC, % neutrophils, total bilirubin, CRP, and NT-proBNP decreased to within the normal range and protein concentration increased (Table 2).
Table 2.
Comparison between pre- and post-IVIG laboratory measures in IVIG-responsive patients
IVIG: intravenous immunoglobulin, WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide
There were 8 patients who became afebrile but had high values of CRP (4.01 to 8.69 mg/dL) and NT-proBNP (338 to 3,308 pg/mL) after IVIG treatment. We repeated the laboratory tests and confirmed their normalization.
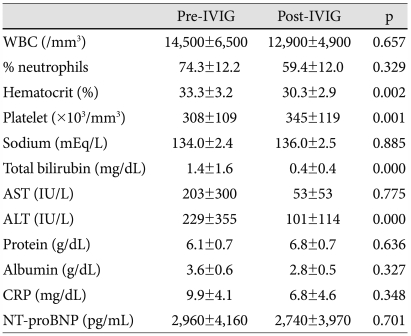
Changes in laboratory measures after intravenous immunoglobulin therapy in resistant patients
There was no difference between pre- and post-IVIG data in WBC, % neutrophils, protein, albumin, CRP, and NT-proBNP. The values of WBC, % neutrophils, CRP, and NT-proBNP remained high after IVIG treatment (Table 3). Paradoxically, WBC counts were elevated in 35% of IVIG-resistant children (vs. 9.4% in responsive patients, p=0.001), % neutrophils in 15% (vs. 4.7% in responsive patients, p=0.15), CRP in 23.8% (vs. 0.9% in responsive patients, p=0.000), and NT-proBNP in 47.4% (vs. 14% in responsive patients, p=0.002) after IVIG therapy, as compared with pre-IVIG data.
Table 3.
Comparison between pre- and post-IVIG laboratory measures in IVIG-resistant patients
IVIG: intravenous immunoglobulin, WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide
In 8 patients who remained febrile after a second dose of IVIG and required pulse steroid therapy, the lab measures before the second dose of IVIG did not significantly differ from those before steroid therapy: WBC, 14,400±6,100 vs. 14,800±3,600, p=0.885; % neutrophils, 65±9 vs. 62±14, p=0.684; CRP, 8.8±5.1 vs. 6.8±3.4, p=0.438; and NT-proBNP, 4,800±5,400 vs. 3,100±3,800, p=0.518.
There were 2 patients who remained febrile despite the second dose of IVIG therapy but had normal laboratory measures, including CRP (1.08 mg/dL and 0.71 mg/dL, respectively). We just observed them, without using the steroid, and they achieved defervescence 24 to 33 hours later.
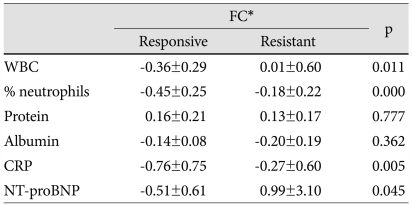
Fractional change of laboratory parameters
We excluded 5 variables that showed a similar change after IVIG treatment between IVIG-responsive and IVIG-resistant patients, and selected the remaining 7 variables that showed a different post-IVIG change between the groups: WBC, % neutrophils, ALT, protein, albumin, CRP, and NT-proBNP. Of these variables, ALT was also excluded from the further analysis because its post-IVIG level in the resistant group decreased toward the normal range.
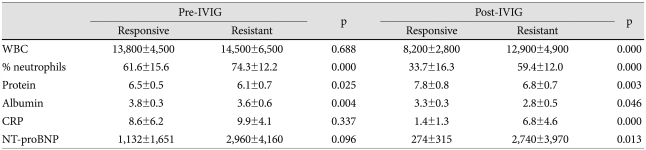
Pre- and post-IVIG concentrations of the 6 variables were compared between the groups (Table 4). There was no difference between the groups in pre-IVIG levels of WBC, CRP, and NT-proBNP. However, the value of % neutrophils was higher and the concentrations of protein and albumin were lower in the IVIG-resistant patients. With regard to post-IVIG data, all 6 variables showed a significant difference between the groups. To verify the possibility that post-IVIG differences between the groups in % neutrophils, protein, and albumin may be derived from their pre-IVIG differences, FCs of the 6 variables were determined (Table 5). As expected from Table 4, WBC, CRP, and NT-proBNP showed a significant FC difference between the groups. Except for protein and albumin, there was a significant inter-group difference in FC of % neutrophils, indicating that its post-IVIG difference seen in Table 4 was not derived from the pre-IVIG difference. Therefore, these 4 variables may represent suitable parameters to guide the decision of retreatment in IVIG-resistant KD.
Table 4.
Comparison of pre- and post-IVIG laboratory measures between IVIG-responsive and IVIG-resistant patients
IVIG: intravenous immunoglobulin, WBC: white blood cell, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide
Table 5.
Fractional changes of laboratory parameters
*FC=(Data at 48 to 72 hours after IVIG)-(Data before IVIG)/Data before IVIG. WBC: white blood cell, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide, FC: fractional changes
Cutoff values of parameters
The cutoff value of parameters was determined by ROC curves: a WBC cutoff value of 10,850/mm3 provided a sensitivity of 80% and a specificity of 84%, with an area under the curve (AUC) of 0.838±0.057 (95% CI, 0.73-0.95, p=0.000); a % neutrophils cutoff value of 53.4% provided a 75% sensitivity and a 87.7% specificity {AUC=0.896±0.031 (95% CI, 0.84-0.96, p=0.000)}; a CRP cutoff value of 3.68 mg/dL yielded 76.2% sensitivity and 92.5% specificity {AUC=0.905±0.041 (95% CI, 0.83-0.99, p=0.000)}; and a NT-proBNP cutoff value of 479 pg/mL yielded 78.9% sensitivity and 86.0% specificity {AUC=0.883±0.05 (95% CI, 0.79-0.98, p=0.000)}.
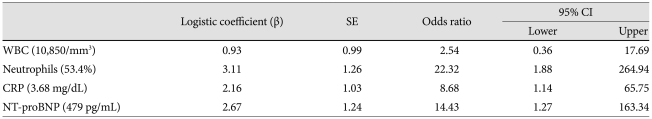
Independent parameters of retreatment
By multivariate logistic regression analysis, % neutrophils, CRP, and NT-proBNP were identified as independent parameters of retreatment (Table 6). Patients with post-IVIG CRP ≥3.68 mg/dL, NT-proBNP ≥479 pg/mL, and/or % neutrophils ≥53.4% were subjected to retreatment, with a sensitivity of 80% and a specificity of 96.3%.
Table 6.
Multivariate logistic analysis for parameters of retreatment
WBC: white blood cell, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide
Discussion
Although IVIG plus aspirin therapy are effective for the treatment of acute KD,1) approximately 10% to 20% of patients ex-perience either persistent or recrudescent fever, despite initial IVIG treatment.2),3) These patients are at increased risk of developing coronary artery aneurysm.2),10),11) Therefore, it is important to determine the effectiveness of IVIG soon after therapy, especially in resistant cases, to recognize a need for additional therapy, such as a second dose of IVIG, steroids, or infliximab.12),13) However, no evaluation system currently exists to determine the effectiveness of IVIG therapy. Both fever resulting from cytokine release and increased CRP levels me-diated by interleukin-6 are clinically useful measures of systemic inflammation in KD. Post-IVIG fever, however, may arise from other causes (e.g., intercurrent infection), as well as from KD itself during hospitalization. Thus other adjuvant inflammatory markers may be required to more precisely assess the ongoing inflammation arising from KD and to support the decision for retreatment in patients who remain febrile after IVIG therapy. We investigated routine laboratory measures and propose herein CRP, NT-proBNP, and % neutrophils as independent parameters to guide the decision of retreatment in KD patients who are still febrile after initial IVIG therapy.
In this study, IVIG-resistance occurred in 17% of KD patients. The clinical indications for retreatment were persistent fever in 14 patients and recrudescent fever in 8 patients. Of these 22 patients retreated with a second dose of IVIG, 14 patients (63.6%) achieved defervescence with this treatment. This finding was similar to results of other studies in which approximately two thirds of the initial non-responders eventually responded to a second dose of IVIG.3),6) In addition, although there was no statistical significance, persistent fever was likely to cease either by a second dose of IVIG or by steroid therapy, whereas recrudescent fever was more likely to cease by a second dose of IVIG only.
The optimal period to begin IVIG administration remains controversial. Some investigators reported that early treatment, i.e., before day 5 of illness, is associated with increased need for additional IVIG therapy.14) Contrarily, others advocated that earlier treatment would be better.15) In this study, we found no difference between the IVIG-responsive and IVIG-resistant group in the proportion of patients who were treated before day 5 of illness (62.0% vs. 77.3%, p=0.215) and also in the mean duration of fever until IVIG initiation. Thus neither group was affected by the time of IVIG administration in this study.
The patients who failed to defervesce after IVIG therapy were characterized by significantly higher values of post-IV-IG WBC, % neutrophils, CRP, and NT-proBNP, compared with IVIG-responsive patients. Thus, we demonstrated that WBC, % neutrophils, CRP, and NT-proBNP may be suitable parameters of retreatment after initial IVIG therapy. When assessed by multivariate analysis, the neutrophil counts and serum concentrations of CRP and NT-proBNP were found to be independent parameters of the response to IVIG therapy. Both the sensitivity and specificity of these 3 independent pa-rameters, as defined in this study, may allow clinicians to identify patients who remain febrile and need additional therapy after initial IVIG treatment. We are able to justify retreatment more reliably by measuring not only CRP, but also NT-proBNP and % neutrophils, rather than CRP alone.
Our results were similar to the findings of Mori et al.9) who demonstrated that increased WBC, neutrophil counts, and CRP after IVIG treatment are markers of treatment efficacy and also useful predictors of CALs. The role of neutrophils in acute KD has been elucidated. Neutrophil activation can occur by stimulation of increased granulocyte colony-stimulating factor and cytokines that are involved in coronary artery dilation.16),17) In the acute phase of disease, enhanced neutrophil function may contribute to the pathogenesis of coronary vascular endothelial injury through either production of elastase or expression of vascular endothelial growth factor.17),18) This was demonstrated in a pathologic study showing early infiltration of neutrophils in the CALs of patients who died 10 days after the onset of disease.19) Thus, it is natural that neutrophil is a marker of systemic inflammation. As a parameter in the multivariate analysis, the neutrophil count is interpreted to be more significant than WBC.
We proposed a CRP cutoff value of 3.68 mg/dL as a marker of ongoing inflammation after IVIG therapy. This value is similar to 4 mg/dL calculated as the upper limit of post-IVIG CRP (mean+2 SDs) in the responsive group, as shown in Table 4, but is still a little higher than the value of 3 mg/dL, proposed by the American Heart Association as a criterion for the evaluation of suspected incomplete KD.20)
We reported previously that NT-proBNP increases during the acute phase of KD, i.e., before IVIG treatment, and decrea-ses to within the normal range in the convalescent phase.21) The change in NT-proBNP according to the phase of KD is equivalent to that in CRP, suggesting that NT-proBNP may be used as a marker of systemic inflammation, as well as myocardial involvement, in acute KD. This finding is consistent with that of the other study.22) Moreover, the fact that, after IVIG administration, NT-proBNP was paradoxically elevated in nearly half of IVIG-resistant patients suggests that NT-proBNP may be a useful marker of ongoing inflammation after IVIG treatment as well. Thus we put an emphasis on the clinical significance of NT-proBNP as a marker of retreatment. A NT-proBNP cutoff value of 479 pg/mL, indicated as a marker of retreatment in the present study, was higher than both 260 pg/mL and 170 pg/mL proposed as a diagnostic test of acute KD in our previous study,21) and in the other study,22) respectively.
Using the defined parameters, here are our suggested guidelines of retreatment for KD patients who receive IVIG treatment: 1) Do necessarily retreat patients who remain febrile despite IVIG therapy and have high values of CRP, NT-proBNP and/or neutrophil counts; 2) When the patients become febrile and show normal lab measures (i.e., parameters), do not retreat for KD and observe for 1 to 2 days, with anticipation of defervescence. In these cases, fever is less likely to be caused by KD; 3) When the patients become afebrile and show abnormal lab values/parameters, do not retreat them and repeat laboratory tests; 4) Do not retreat patients who become afebrile and have normal parameters.
In conclusion, our study shows that, in KD patients who remain febrile despite IVIG treatment, additional therapy can be facilitated early by measuring CRP in combination with NT-proBNP and/or % neutrophils. Further study in a larger sample of patients is warranted to clarify our guidelines of retreatment for KD patients.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 2.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 4.Reller M, DeCristofaro J, Schwartz DC. Coronary aneurysms in a patient with atypical Kawasaki syndrome and a streptococcal infection. Pediatr Cardiol. 1984;5:205–207. doi: 10.1007/BF02427046. [DOI] [PubMed] [Google Scholar]
- 5.Johnson D, Azimi P. Kawasaki disease associated with Klebsiella pneumoniae bacteremia and parainfluenza type 3 virus infection. Pediatr Infect Dis. 1985;4:100. doi: 10.1097/00006454-198501000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin retreatment in Kawasaki disease. J Pediatr. 1993;123:657–659. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 7.Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 8.Research committee on Kawasaki disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Ministry of Health and Welfare; 1984. [Google Scholar]
- 9.Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J Pediatr. 2000;137:177–180. doi: 10.1067/mpd.2000.107890. [DOI] [PubMed] [Google Scholar]
- 10.Fukunishi M, Kikkawa M, Hamana K, et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr. 2000;137:172–176. doi: 10.1067/mpd.2000.104815. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 12.Burns JC, Mason WH, Hauger SB, et al. Infliximab treatment for refractory Kawasaki syndrome. J Pediatr. 2005;146:662–667. doi: 10.1016/j.jpeds.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Song MS, Lee SB, Sohn S, et al. Infliximab treatment for refractory Kawasaki disease in Korean children. Korean Circ J. 2010;40:334–338. doi: 10.4070/kcj.2010.40.7.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muta H, Ishii M, Egami K, et al. Early intravenous gamma-globulin treatment for Kawasaki disease: the nationwide surveys in Japan. J Pediatr. 2004;144:496–499. doi: 10.1016/j.jpeds.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Tse SM, Silverman ED, McCrindle BW, Yeung RS. Early treatment with intravenous immunoglobulin in patients with Kawasaki disease. J Pediatr. 2002;140:450–455. doi: 10.1067/mpd.2002.122469. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Noda E, Miyawaki M, Tacheuchi T, Uemura S, Koike M. Serum levels of neutrophil activation cytokines in Kawasaki disease. Pediatr Int. 2001;43:115–119. doi: 10.1046/j.1442-200x.2001.01362.x. [DOI] [PubMed] [Google Scholar]
- 17.Samada K, Igarashi H, Shiraishi H, Hatake K, Momoi MY. Increased serum granulocyte colony-stimulating factor correlates with coronary artery dilatation in Kawasaki disease. Eur J Pediatr. 2002;161:538–541. doi: 10.1007/s00431-002-1018-5. [DOI] [PubMed] [Google Scholar]
- 18.Hamamichi Y, Ichida F, Yu X, et al. Neutrophils and mononuclear cells express vascular endothelial growth factor in acute Kawasaki disease: its possible role in progression of coronary artery lesions. Pediatr Res. 2001;49:74–80. doi: 10.1203/00006450-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Oharaseki T, Naoe S, Wakayama M, Yokouchi Y. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int. 2005;47:305–310. doi: 10.1111/j.1442-200x.2005.02049.x. [DOI] [PubMed] [Google Scholar]
- 20.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Kim H, Kim HS, Sohn S. NT-pro BNP: a new diagnostic screening tool for Kawasaki disease. Korean J Pediatr. 2006;49:539–544. [Google Scholar]
- 22.Dahdah N, Siles A, Fournier A, et al. Natriuretic peptide as an adjunctive diagnostic test in the acute phase of Kawasaki disease. Pediatr Cardiol. 2009;30:810–817. doi: 10.1007/s00246-009-9441-2. [DOI] [PubMed] [Google Scholar]