Scheme 3.
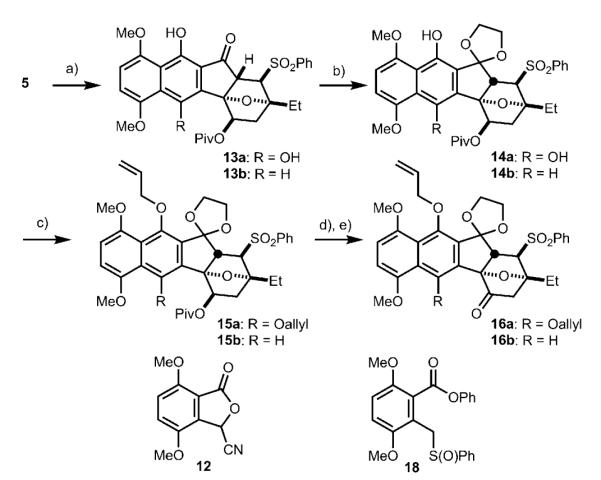
Synthesis of dimerization precursors 16 a and 16 b. a)For 13 a: 12, LHMDS, HMPA, THF, −78°C, 30 min; then 5, 50°C, 3 h, 85 %; for 13 b: 18, LHMDS, THF, −60°C, 6 h; then 5, −78°C, 10 min, 79 %; b) 1,2-bis(trimethylsilyloxy) ethane, TMSOTf, CH2Cl2, 23°C, 48 h, 76 % for 14 a, 72 % for 14 b; c) Cs2CO3, allyl bromide, DMF, 23°C, 2 h, 87 % for 15 a, 76 % for 15 b; d) NaBHEt3, THF, 0 °C, 4 h; e) TPAP, NMO, 4 Å M.S., CH2Cl2, 23°C, 2 h, 90 % for two steps for 16a, 88% for two steps for 16b. HMPA = hexamethylphosphoramide, DMF= dimethyl formamide, TMSOTf = trimethylsilyl trifluoromethanesulfonate, TPAP = tetrapropylammonium perruthenate, NMO = 4-methylmorpholine-N-oxide.
