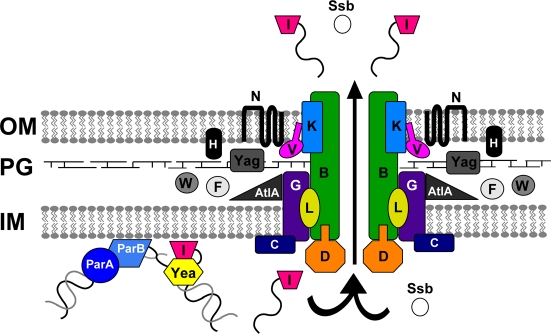
Figure 3.
Model of type IV secretion in gonococci. Predicted functions can be assigned to many of the proteins encoded by the GGI based on their similarity to other characterized type IV secretion system proteins. The putative partitioning proteins ParA and ParB may bring the DNA to the apparatus. TraI nicks the DNA at the origin of transfer (oriT), and a helicase, possibly Yea, unwinds it. The relaxase likely remains bound to the 5′ end of the DNA and pilots it to the secretion apparatus, where it may dock with the putative coupling protein, TraD. The putative ATPase TraC and the mating-pair stabilization homolog TraG likely contribute to the inner membrane pore. Periplasmic proteins such as Yag, TraW, TraF, TraH, and AtlA are likely involved in apparatus assembly or in making localized breaks in the peptidoglycan layer. The DNA is then secreted through the transmembrane apparatus, the core proteins of which are predicted to be TraB, TraK, and TraV. TraN may form part of the outer membrane pore. The DNA is secreted into the extracellular environment. Proteins such as single-stranded binding protein (Ssb) may also be secreted, but there is no evidence as-yet for protein secretion by the gonococcal T4SS. OM, outer membrane; PG, peptidoglycan; IM, inner membrane.