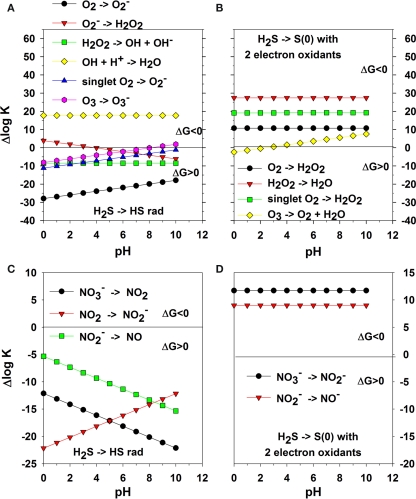
Figure 1.
One-electron transfer reactions of H2S with oxygen species; the + Δlog K on the y-axis indicates a favorable complete reaction and − Δlog K indicates an unfavorable reaction as ΔGo = −RT lnK = −2. 303 RT log K. HS− reactions are not included but are similar in reactivity (note pK of H2S ∼7 and depends on salinity and temperature). (A) one-electron transfer reactions of oxygen species coupled with Eq. 5. (B) two-electron transfer reactions of oxygen species coupled with Eq. 8. (C) one-electron transfer reactions of oxidized nitrogen species coupled with Eq. 5. (D) two-electron transfer reactions of oxidized nitrogen species coupled with Eq. 8.