Summary
Deficiency of von Willebrand factor (VWF) cleaving protease ADAMTS13 has been demonstrated to be the proximate cause of a subset of thrombotic microangiopathic haemolytic anaemias (MAHA) typical for thrombotic thrombocytopenic purpura (TTP). ADAMTS13 gene mutations cause the hereditary form; acquired deficiency has been attributed to presence of an autoantibody, which may represent a specific subset of MAHA best termed ‘autoimmune thrombotic thrombocytopenic purpura’. We describe a patient with relapsing TTP because of ADAMTS13 inhibitors, who failed to achieve sustained remission despite therapies with plasma exchange, steroids, vincristine, staphylococcal protein A and splenectomy. The ADAMTS13 inhibitor titre remained elevated and clinical stability was only maintained by plasma exchange every 2–3 d over a period of 268 d. The patient then received rituximab therapy (eight doses of 375 mg/m2 weekly), during which she required five plasma exchanges in the first 10 d, two exchanges in the next 3 weeks, and none thereafter for 450 d and ongoing. The ADAMTS13 inhibitor titre decreased and enzyme activity increased. We compared this case with that of seven previously reported TTP cases also treated with rituximab; experience suggests that rituximab therapy deserves further investigation for patients with either refractory or relapsing TTP caused by ADAMTS13 inhibitors.
Keywords: thrombotic thrombocytopenic purpura, rituximab, plasma exchange
Thrombotic thrombocytopenic purpura (TTP), a relatively uncommon but serious disease characterized by the development of von Willebrand factor (VWF)-platelet rich hyaline thrombi in the arterioles and capillaries, affects the brain, heart, pancreas, kidney and other organs (Bukowski, 1982). Complications from neurological or cardiac abnormalities are the most common cause of death.
The use of plasma exchange has decreased the mortality rate of TTP from >90% to <10–30% (Rock et al, 1991). However, relapse of the disease occurs in more than one-third of the cases that achieve remission (Shumak et al, 1995; Bell, 1997). A subset of patients develop multiple relapses or persistent disease, which requires numerous sessions of plasma exchange; this is costly, technically demanding, often associated with serious complications such as infection, thrombosis and/or transfusion reactions, and is debilitating to the patients. Some of the cases that fail to achieve a sustained remission with plasmapheresis improve after the use of corticosteroids, vincristine, cyclophosphamide, azathioprine, high dose intravenous immunoglobulin, cyclosporin A, staphylococcal protein A immunoadsorption and/or splenectomy (Gaddis et al, 1997; Kwaan & Soff, 1997; Allford et al, 2003; Aqui et al, 2003). However, other patients are unresponsive to these therapies and require long-term plasma exchange to prevent a fatal outcome.
Recent studies demonstrate that a subset of thrombotic microangiopathic haemolytic anaemias (MAHA) is associated with severe deficiency of VWF-cleaving protease ADAMTS13. Mutations in the ADAMTS13 gene have been detected in cases presenting as Schulman–Upshaw syndrome (Levy et al, 2001; Kokame et al, 2002; Schneppenheim et al, 2003). However, deficiency of the ADAMTS13 enzyme in adolescents and adults is most often due to an immunoglobulin (Ig)G autoantibody against the ADAMTS13 enzyme (Furlan et al, 1998; Tsai & Lian, 1998). The autoimmune cause of the deficiency suggests that these cases may respond to immunosuppressive therapies.
Rituximab, a chimaeric monoclonal antibody against CD20 that depletes B cells in the circulation and lymphoid tissues, has shown efficacy in the treatment of CD20-positive lymphoproliferative disorders (Boye et al, 2003). The agent has also been used with success in patients with immune thrombocytopenic purpura and other types of autoimmune diseases (Levine & Pestronk, 1999; Berentsen et al, 2001; Specks et al, 2001; Stasi et al, 2001; Dimopoulos et al, 2002; Mori et al, 2002; Motto et al, 2002; Perrotta et al, 2002; Remuzzi et al, 2002; Salopek et al, 2002; Zaja et al, 2002, 2003a,b; Renaud et al, 2003; Trape et al, 2003; Zecca et al, 2003). In preliminary studies, patients with refractory TTP have achieved sustained remission after rituximab therapy (Chemnitz et al, 2002; Gutterman et al, 2002; Tsai & Shulman, 2003; Zheng et al, 2003).
We describe the experience of using rituximab in a case of refractory TTP and compare the experience with previously reported cases.
Case report
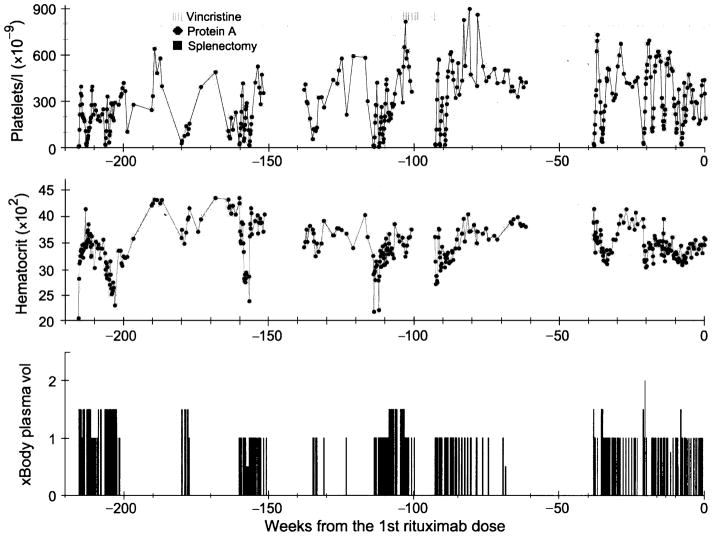
The patient, an African American female, developed her first episode of TTP at the age of 26 years, when she presented with confusion and lethargy. Her platelet count was 8 × 109/l; haemoglobin, 6·0 g/dl; creatinine, 106 μmol/l and lactate dehydrogenase (LDH), 1275 IU/l (normal <250 IU/l). Blood smears contained schistocytes. A diagnosis of TTP was made and plasma exchange was started. Remission was achieved after 59 sessions of plasma exchange and four doses of vincristine over a period of 99 d (Fig 1). In the following 4 years, she experienced five relapses of TTP, each requiring eight to 91 plasma exchanges over 19 to 270 d respectively (Fig 1). During that period, she also received four treatments of immunoadsorption therapy with staphylococcal protein A columns (Prosorba®; Fresenius AG, Bad Homberg, Germany), a splenectomy and seven doses of vincristine. After her last relapse on day 268 before initiation of rituximab therapy, she became dependent on plasma exchange; whenever plasma exchange was withheld, her platelet count decreased to very low levels within 2–3 d (Fig 2). Cyclophosphamide, azathioprine or cyclosporin A was not used because of the patient’s concern of potential adverse effects. On several occasions the patient had suffered adverse reactions, such as hives and rigours, associated with plasma transfusion and complications of bleeding, and infection and thrombosis in or around the central lines.
Fig 1.
Platelet count, haematocrit and plasma exchange (× body plasma volume) data prior to the initiation of rituximab therapy. The use of vincristine or staphylococcal protein A columns and the date of splenectomy are indicated.
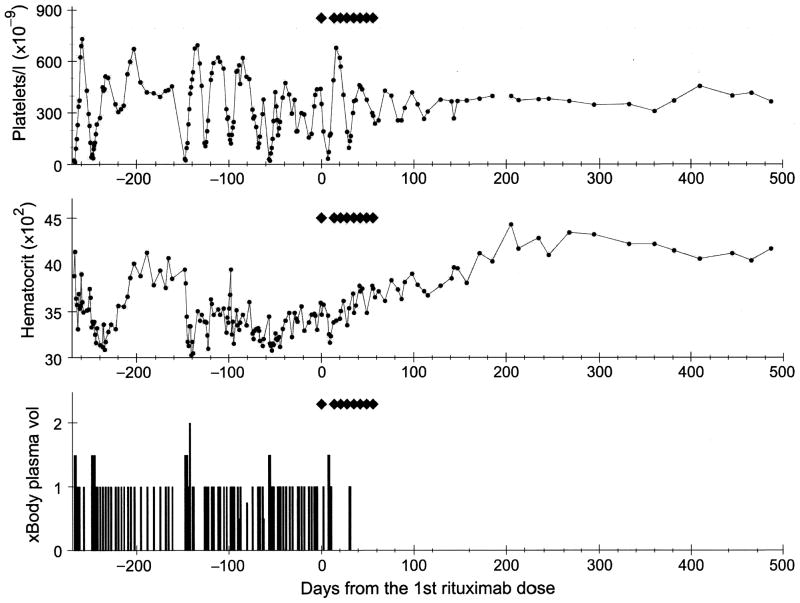
Fig 2.
Platelet count, haematocrit and plasma exchange data immediately prior to the initiation of rituximab and subsequent follow-up. Each ◆ indicates one dose of rituximab (375 mg/m2).
On a compassionate use basis, rituximab (Genentech Inc., San Francisco, CA, USA), 375 mg/m2, was initiated. Intravenous doses (eight) were administered weekly; each dose was accompanied by acetaminophen (650 mg) and diphenhydramine (25 mg). There were no adverse reactions in association with the drug administration. She required five plasma exchanges in 10 d following the first dose of rituximab and two additional plasma exchanges in the next 3 weeks. In each instance, rituximab was administered following plasma exchange. Plasma exchange was performed no sooner than 48 h following a dose of rituximab. Indeed, the second weekly dose of rituximab was held an additional week because of the need to continue five sessions of plasma exchange following the first dose. Since then, her platelet count has stabilized within the normal range. Her haemoglobin and LDH levels gradually normalized and the follow-up smear showed a mild postsplenectomy picture but is otherwise normal. She has not required plasma exchange for 450 d and this is ongoing (Fig 2). The patient has not presented any evidence of other autoimmune diseases; the human immunodeficiency virus, hepatitis B and C serology tests have been negative. She has no history of taking any medications known to be associated with TTP.
Materials and methods
With informed consent from the patient, plasma samples were obtained by centrifugation of whole blood in 3·2% sodium citrate and stored at −70°C. Determination of ADAMTS13 activity in a plasma sample was based on the generation of homodimers of the 176- and 140-kDa fragments of the VWF substrate as previously described (Tsai & Lian, 1998). A test plasma sample was mixed with a normal control sample to detect ADAMTS13 inhibitor activity. The Bethesda unit, originally defined for measuring the inhibitor titre of factor VIII (Tsai et al, 2001), was used to determine the inhibitor titre of ADAMTS13. One unit of inhibitor suppresses ADAMTS13 activity by 50% in an equal mixture of normal and patient plasma after 1 h of incubation at 37°C. VWF multimers were analysed by sodium dodecyl sulphate agarose gel electrophoresis (Tsai & Lian, 1998). The Institutional Review Boards at the participating facilities approved the study protocols.
Results
ADAMTS13 analysis
ADAMTS13 activity was determined on 22 occasions before rituximab was initiated. It was 0–0·1 U/ml (normal range 0·79–1·27 U/ml) on eight occasions when her platelet count was below 50 × 109/l or decreased by 50% from a previously stable level. The protease level was 0·1–0·8 U/ml on 13 occasions when she had received plasma exchange and her platelet count was rising, and was 0·13 U/ml on one occasion when she was in remission.
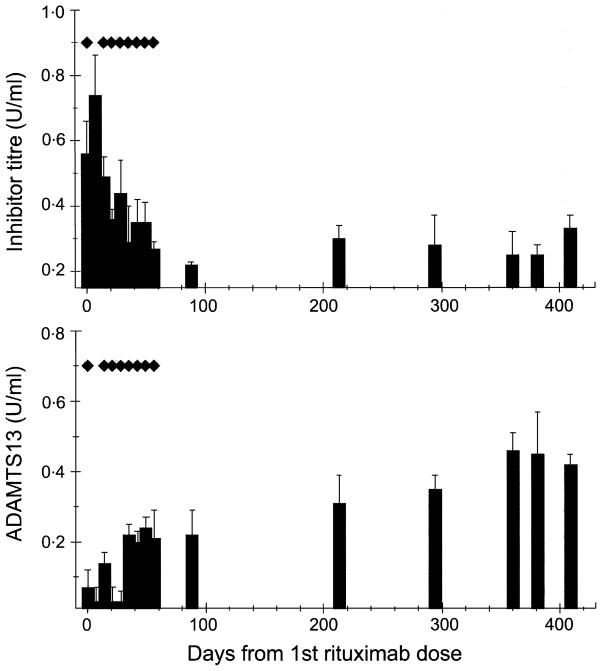
The course of her ADAMTS13 activity level and the inhibitor titre from the day she received the first dose of rituximab are depicted in Fig 3. During the first 31 d, the protease activity level was measured on five occasions. It was less than 0·1 U/ml except on one occasion, 4 d after plasma exchange was completed, when it was 0·14 U/ml. On day 36, the ADAMTS13 activity level increased to 0·22 U/ml and, thereafter, has been consistently higher than the levels measured prior to rituximab therapy initiation (P < 0·001 by ANOVA). The ADAMTS13 inhibitor titre was 0·56 U/ml before treatment with rituximab. It decreased to 0·29 U/ml (P < 0·01 by ANOVA) on day 36 and on subsequent determinations has remained between 0·22 and 0·35 U/ml (normal <0·2 U/ml).
Fig 3.
ADAMTS13 inhibitor titres and activity levels (mean ± SD) after the initiation of rituximab therapy. Each ◆ indicates one dose of rituximab (375 mg/m2). The inhibitor titre first showed a decrease by day 21 P < 0·05), while the ADAMTS13 activity level first increased by day 35 and remained in the range of 0·2–0·4 U/ml (P < 0·01).
Review of the literature
Four previous reports have described the use of rituximab in seven cases of TTP (Chemnitz et al, 2002; Gutterman et al, 2002; Tsai & Shulman, 2003; Zheng et al, 2003). The clinical features and the response to rituximab of these and the present case are summarized in Tables I and II. In all instances the patients were females, aged 31–62 years.
Table I.
Features of the cases of TTP treated with rituximab.
| Study | Case no. | Age (years) | Sex (M, F) | Duration of TTP (years) | Other therapies* | Active disease leading to rituximab
|
|
|---|---|---|---|---|---|---|---|
| Duration (d) | Plasma exchange (no.) | ||||||
| Gutterman et al (2002) | 1 | 54 | F | 4·75 | 1, 2, 4, 7, 8 | 166 | 102 |
| 2 | 62 | F | 10 | 1, 2, 7, 8 | 223 | 44 | |
| 3 | 40 | F | 3 | 2, 3, 5, 7, 8 | 50 | 42 | |
| Chemnitz et al (2002) | 1 | 39 | F | 12 d | – | 12 | 12 |
| 2 | 37 | F | 7 d | – | 7 | 7 | |
| Zheng et al (2003) | 1 | 42 | F | 2·3 | 7, 8 | 10 | 10 |
| Tsai and Shulman (2003) | 1 | 36 | F | 9·3 | 2, 3, 8 | 455 | 0 |
| This report | 1 | 31 | F | 4·1 | 2, 6, 7, 8 | 270 | 94 |
| Overall | 8 | 31–62 | M: 0 | Acute: 7–12 d | Six cases | Acute: 7–12 | Acute: 7–12 |
| F: 8 | Chronic: 2·3–10 | Chronic: 10–455 | Chronic: 10–102 | ||||
In addition to plasma exchange.
1, azathioprine (two cases); 2, corticosteroids (five cases); 3, cyclophosphamide (two cases); 4, cyclosporin A (one case); 5, intravenous immunoglobulins (one case); 6, protein A columns (one case); 7, splenectomy (five cases); 8, vincristine (six cases).
TTP, thrombotic thrombocytopenic purpura.
Table II.
Dose of rituximab and clinical response.
| Study | Case no. | Rituximab dose (375 mg/m2) | Concurrent therapies | Clinical remission* | Remission duration (months) |
|---|---|---|---|---|---|
| Gutterman et al (2002) | 1 | 8 | None | Yes | 36+ |
| 2 | 8 | None | Yes | 17† | |
| 3 | 4 | None | No‡ | – | |
| Chemnitz et al (2002) | 1 | 4 | Vincristine | Yes | 2§ |
| Corticosteroids | Yes | 12+ | |||
| 2 | 2 | Vincristine | |||
| Corticosteroids | |||||
| Zheng et al (2003) | 1 | 6 | Cyclophosphamide | Yes | 10+ |
| Tsai and Shulman (2003) | 1 | 8 | None | Yes | 24+ |
| This report | 1 | 8 | None | Yes | 15+ |
| Overall | 8 | 2–8 | Three cases | Seven cases | 2–36 |
Defined as normal platelet count, stable haemoglobin level, and no symptoms or signs that are potentially caused by thrombotic thrombocytopenic purpura.
Platelet count increased and required less-intensive plasma exchanges.
Relapse.
Lost to follow-up.
One report describes the use of rituximab in two cases during their first episodes of TTP (Chemnitz et al, 2002); the other six (including this case) had multiple relapses over a period of 2·3–10 years. They all had failed other modalities of treatment, including corticosteroids, azathioprine, cyclophosphamide, cyclosporin A, intravenous immunoglobulins, protein A immunoadsorption, splenectomy and vincristine. The indications for use of rituximab included: (i) failure to respond to plasma exchange (two cases, both in their first episodes), (ii) multiple relapses (one case), (iii) long-term plasma exchange (four cases requiring 42–102 exchanges over a period of 50–223 d) and (iv) recurrent episodes of cerebral ischaemia (one case). The patient treated for recurrent episodes of cerebral ischaemia had normal blood cell counts for 455 d preceding the treatment. All seven patients demonstrated ADAMTS13 deficiency because of the presence of inhibitors prior to rituximab therapy (Table III). Intravenous rituximab was administered at a dose of 375 mg/m2/week for 2–8 weeks. No serious adverse reactions were reported. In five cases rituximab was the only therapy during the course of treatment. In the two cases treated during the first episode and the case with relapsing TTP additional therapies were administered during the course of rituximab therapy (vincristine and corticosteroids in the two acute cases and cyclophosphamide in the case treated for multiple relapses).
Table III.
ADAMTS13 and its inhibitors before and after rituximab therapy.
| Study | Case no. | ADAMTS13 activity and inhibitors
|
|||
|---|---|---|---|---|---|
| Before rituximab
|
After rituximab
|
||||
| Activity | Inhibitor | Activity | Inhibitor titre | ||
| Gutterman et al (2002) | 1 | ND | ND | – | – |
| 2 | Deficiency | Yes | Increased | Decreased | |
| 3 | Deficiency | Yes | Unchanged | Decreased | |
| Chemnitz et al (2002) | 1 | Deficiency | Yes | Increased | Decreased |
| 2 | ND | ND | ND | ND | |
| Zheng et al (2003) | 1 | Deficiency | Yes | Increased | Decreased |
| Tsai and Shulman (2003) | 1 | Deficiency | Yes | Increased | Decreased |
| This report | 1 | Deficiency | Yes | Increased | Decreased |
| Overall | 8 | Deficiency: 6/6 | 6/6 | Increased: 5/6 | Decreased: 5/5 |
ND, not done. Subsequent investigation revealed the presence of ADAMTS13 deficiency because of inhibitors.
Seven cases achieved remission. Time to remission with sustained normal platelet counts was 2–5 weeks. The one case that did not achieve remission nevertheless responded with an increased platelet count and also required less-intensive plasma exchange. This case received four doses of rituximab, and plasma exchange was continued throughout the course of treatment.
Among the patients who achieved remission, the duration of response was 1·7 years in one case and 10–36 months and ongoing in the remaining five cases. One case was lost to follow up at 2 months.
A direct comparison of the response of ADAMTS13 activity level and its inhibitor among these reports is not possible because different types of assays were used, as recently reviewed (Tsai, 2003). Nevertheless, evidence of lower inhibitor titres following rituximab therapy was observed in all six cases that were investigated both pre- and postrituximab therapy (Table III). Among these six cases, five had evidence of increased ADAMTS13 activity levels. The only case that did not show evidence of increased ADAMTS13 activity levels also did not achieve remission. Notably, none of the patients with chronic TTP had a sustained normalization of ADAMTS13 activity levels.
Discussion
Stabilization of platelet counts and cessation of haemolysis occurred after rituximab therapy in a patient with relapsing TTP, who had previously required plasma exchange every 2–3 d, over a period of 9 months, for treatment of the disease. The patient required five exchanges in the first 10 d after the first dose of rituximab, two exchanges between days 11 and 31, and has not required treatment thereafter (>450 d).
Analysis of ADAMTS13 activity detected a severe deficiency of the protease and the presence of an IgG inhibitor throughout her course. Her inhibitor titre was in the range typically observed in patients with TTP (Tsai et al, 2001). Before rituximab therapy, her protease activity level was persistently ≤0·10 U/ml when she was not being treated with plasma exchange. After rituximab therapy, her inhibitor titre decreased, accompanied by an increase in ADAMTS13 activity levels. Nevertheless, during her long period of remission, ADAMTS13 inhibitor activity remained detectable and the enzyme activity remained subnormal, suggesting that rituximab therapy induced clinical remission in this case by diminishing, but not eliminating, the autoantibody against ADAMTS13.
A review of the literature revealed that the response of this case to rituximab is similar to that of previously reported cases of chronic TTP (Gutterman et al, 2002; Tsai & Shulman, 2003; Zheng et al, 2003). From all reported cases treated with rituximab, two had acute TTP, and rituximab was introduced within 2 weeks of the initial presentation (Chemnitz et al, 2002). Because remission is common when acute TTP is treated with plasma exchange for 7–14 d, the response of these two patients to rituximab is difficult to evaluate. In a third patient, the concurrent use of cyclophosphamide soon after a relapse also diminishes the confidence that remission was due to rituximab. However, anecdotal experience suggests that a response to cyclophosphamide is uncommon within a few weeks. The remaining five cases suffered multiple relapses over a course of 2·3–10 years and active or relapsing disease lasting from 50 d to 15 months preceding the use of rituximab. Among these, a coincidence of remission independent of rituximab therapy in each case is extremely unlikely, although not completely impossible.
Role of other therapies
Previously, because the pathogenesis of TTP was unknown, patients with relapsing TTP were empirically treated with corticosteroids, vincristine, cyclophosphamide, azathioprine, splenectomy, cyclosporin A, intravenous immunoglobulins or staphylococcal protein A columns (Prosorba®) (Gaddis et al, 1997; Kwaan & Soff, 1997; Allford et al, 2003; Aqui et al, 2003). However, the efficacy of these treatments has not been systemically evaluated. Among these eight reported cases, excluding the two cases with their initial episodes of TTP and one case that was treated concurrently with cyclophosphamide, all had failed other modalities of treatment before the introduction of rituximab as the only treatment. The experience of these cases suggests that rituximab therapy is effective among patients who have failed other modalities of therapies, although the role of rituximab and other modalities in the treatment of TTP remains to be determined in future studies.
Dosage and administration
Rituximab is generally administered as four weekly doses for the treatment of lymphoproliferative diseases. However, eight weekly doses have been used with no additional adverse reactions (Piro et al, 1999). The number of doses used in patients with TTP ranged from two to eight. Although removal of the administered agent during plasmapheresis is a concern, data of serum rituximab levels among patients undergoing plasma exchange are not available, and it is not clear whether plasma exchange on day 1 or a few days after the intravenous administration of rituximab has a negative impact on the therapeutic efficacy of the antibody. Notably, the present case received multiple plasma exchanges beginning 2 d after the first dose of rituximab. Nevertheless, she required only two exchanges after the second dose. The experience of this case suggests that perhaps because of a rapid extravascular distribution, plasma exchange one or a few days after completion of rituximab administration may not have a major negative impact. However, the only case that did not achieve remission also happened to receive plasma exchange throughout her period of rituximab therapy, raising the suspicion that removal of the administered antibody might have contributed to her failure to respond. Until more data are available to address the concern of a potentially negative impact, it seems reasonable to postpone plasma exchange as long as possible after the rituximab dose. However, plasma exchange should not be withheld if it is deemed clinically necessary. Clearly, further studies are needed to determine whether supplemental doses of rituximab after plasma exchange are needed to compensate for the loss of the antibody during plasma exchange and, thus, promote clinical response.
Time to response
In this patient, a diminished need for plasma exchange was apparent within 2 weeks after the first dose of rituximab; she required five plasma exchanges in the first 10 d after rituximab was initiated, but only two plasma exchanges in the next 20 d. An evaluation of the responses observed in other cases suggests that, within 2–5 weeks after administration of the first dose of rituximab, alleviation of disease activity and a decrease in the need for plasma exchange are observed.
Duration of response and risk of relapse
Previous studies show that rituximab administration is followed by depletion of B cells in the circulation and in the lymphoid tissues (Kneitz et al, 2002; Zaja et al, 2002, 2003a,b). Recovery of the B cells is typically apparent by 6–12 months. In TTP patients treated with rituximab, one case had a relapse at 17 months. Another case showed a gradual decrease in the ADAMTS13 activity level starting from month 14 after the treatment was started, although she remains in remission for 3 years and on going (H. M. Tsai, unpublished observations). This pattern suggests that relapse is possible. As relapse can be devastating or fatal, further investigation is needed to delineate whether and when retreatment should be considered.
Risks and benefits
A prolonged course of plasma exchange is expensive, debilitating to the patients, and often associated with the development of infection or other complications related to venous access lines. Adverse reactions to plasma are also common. Although rituximab may be associated with serious reactions, most adverse events, including chills, fever and rigours, are infusion-related and can be avoided or alleviated by following appropriate guidelines. Serious cardiopulmonary or mucocutaneous reactions have been also been reported. Therefore, the risk of rituximab therapy should be carefully evaluated against the risk of plasma exchange or alternative therapies.
Potential role of rituximab in the management of acute TTP
Some patients with acute TTP are refractory to plasma exchange, resulting in a fatal outcome. Other patients develop relapses after achieving an initial remission. It is tempting to speculate that rituximab, by suppressing the B-cell immune response, may also facilitate remission among patients with acute TTP and diminish the risk of subsequent relapses. However, the course of TTP is typically unpredictable and remission in anecdotal cases that receive rituximab therapy cannot be considered with certainty as a response to the treatment. A randomized control study would be needed before rituximab could be recommended as an adjuvant to plasma exchange.
In summary, the experience of this and previous cases demonstrates that rituximab treatment is followed by sustained remission in two cases of acute TTP and five of six cases of chronic TTP. Rituximab increased the ADAMTS13 levels and decreased, but did not eliminate, the inhibitor of ADAMTS13 among patients with chronic TTP. Thus, patients with chronic refractory TTP appear to be good candidates for treatment with rituximab because a small increase of the ADAMTS13 activity level is apparently sufficient for inducing long-term clinical remission. Based on these preliminary results, a systematic clinical trial designed to determine the precise role and efficacy of rituximab in the treatment and management of refractory TTP is urgently needed. Such a trial would, for example, provide data on the utility of whether serial ADAMTS13 assays are predictive for response or subsequent risk of TTP relapse. In the interim, based on the compilation of reported cases and our experience, the use of rituximab appears to be a reasonable strategy in otherwise highly refractory cases of TTP.
Acknowledgments
This study was supported in part by a grant (HL R01 62136 to HMT) from the National Heart, Lung and Blood Institute of the NIH.
References
- Allford SL, Hunt BJ, Rose P, Machin SJ. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. British Journal of Haematology. 2003;120:556–573. doi: 10.1046/j.1365-2141.2003.04049.x. [DOI] [PubMed] [Google Scholar]
- Aqui NA, Stein SH, Konkle BA, Abrams CS, Strobl FJ. Role of splenectomy in patients with refractory or relapsed thrombotic thrombocytopenic purpura. Journal of Clinical Apheresis. 2003;18:51–54. doi: 10.1002/jca.10053. [DOI] [PubMed] [Google Scholar]
- Bell WR. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome relapse: frequency, pathogenesis, and meaning. Seminars in Hematology. 1997;34:134–139. [PubMed] [Google Scholar]
- Berentsen S, Tjonnfjord GE, Brudevold R, Gjertsen BT, Langholm R, Lokkevik E, Sorbo JH, Ulvestad E. Favourable response to therapy with the anti-CD20 monoclonal antibody rituximab in primary chronic cold agglutinin disease. British Journal of Haematology. 2001;115:79–83. doi: 10.1046/j.1365-2141.2001.03078.x. [DOI] [PubMed] [Google Scholar]
- Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Annals of Oncology. 2003;14:520–535. doi: 10.1093/annonc/mdg175. [DOI] [PubMed] [Google Scholar]
- Bukowski RM. Thrombotic thrombocytopenic purpura: a review. Progress in Hemostasis and Thrombosis. 1982;6:287–337. [PubMed] [Google Scholar]
- Chemnitz J, Draube A, Scheid C, Staib P, Schulz A, Diehl V, Sohngen D. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. American Journal of Hematology. 2002;71:105–108. doi: 10.1002/ajh.10204. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Zervas C, Zomas A, Hamilos G, Gika D, Efstathiou E, Panayiotidis P, Vervessou E, Anagnostopoulos N, Christakis J. Extended rituximab therapy for previously untreated patients with Waldenstrom’s macroglobulinemia. Clinical Lymphoma. 2002;3:163–166. doi: 10.3816/clm.2002.n.022. [DOI] [PubMed] [Google Scholar]
- Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. New England Journal of Medicine. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- Gaddis TG, Guthrie TH, Jr, Drew MJ, Sahud M, Howe RB, Mittelman A. Treatment of plasma refractory thrombotic thrombocytopenic purpura with protein A immunoabsorption. American Journal of Hematology. 1997;55:55–58. doi: 10.1002/(sici)1096-8652(199706)55:2<55::aid-ajh1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Gutterman L, Kloster B, Tsai HM. Rituximab therapy for refractory thrombotic thrombocytopenic purpura. Blood Cells, Molecules, and Diseases. 2002;28:385–391. doi: 10.1006/bcmd.2002.0522. [DOI] [PubMed] [Google Scholar]
- Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune diseases. Immunobiology. 2002;206:519–527. doi: 10.1078/0171-2985-00200. [DOI] [PubMed] [Google Scholar]
- Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y, Miyata T, Fujimura Y. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaan HC, Soff GA. Management of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Seminars of Hematology. 1997;34:159–166. [PubMed] [Google Scholar]
- Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using rituximab. Neurology. 1999;52:1701–1704. doi: 10.1212/wnl.52.8.1701. [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- Mori A, Tamaru J, Sumi H, Kondo H. Beneficial effects of rituximab on primary cold agglutinin disease refractory to conventional therapy. European Journal of Haematology. 2002;68:243–246. doi: 10.1034/j.1600-0609.2002.01667.x. [DOI] [PubMed] [Google Scholar]
- Motto DG, Williams JA, Boxer LA. Rituximab for refractory childhood autoimmune hemolytic anemia. Israel Medical Association Journal. 2002;4:1006–1008. [PubMed] [Google Scholar]
- Perrotta S, Locatelli F, La Manna A, Cennamo L, De Stefano P, Nobili B. Anti-CD20 monoclonal antibody (rituximab) for life-threatening autoimmune haemolytic anaemia in a patient with systemic lupus erythematosus. British Journal of Haematology. 2002;116:465–467. [PubMed] [Google Scholar]
- Piro LD, White CA, Grillo-Lopez AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Annals of Oncology. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- Renaud S, Gregor M, Fuhr P, Lorenz D, Deuschl G, Gratwohl A, Steck AJ. Rituximab in the treatment of polyneuropathy associated with anti-MAG antibodies. Muscle and Nerve. 2003;27:611–615. doi: 10.1002/mus.10359. [DOI] [PubMed] [Google Scholar]
- Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. New England Journal of Medicine. 1991;325:393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- Salopek TG, Logsetty S, Tredget EE. Anti-CD20 chimeric monoclonal antibody (rituximab) for the treatment of recalcitrant, life-threatening pemphigus vulgaris with implications in the pathogenesis of the disorder. Journal of the American Academy of Dermatology. 2002;47:785–788. doi: 10.1067/mjd.2002.126273. [DOI] [PubMed] [Google Scholar]
- Schneppenheim R, Budde U, Oyen F, Angerhaus D, Aumann V, Drewke E, Hassenpflug W, Haberle J, Kentouche K, Kohne E, Kurnik K, Mueller-Wiefel D, Obser T, Santer R, Sykora KW. Von Willebrand factor cleaving protease and ADAMTS13 mutations in childhood TTP. Blood. 2003;101:1845–1850. doi: 10.1182/blood-2002-08-2399. [DOI] [PubMed] [Google Scholar]
- Shumak KH, Rock GA, Nair RC. Late relapses in patients successfully treated for thrombotic thrombocytopenic purpura. Canadian Apheresis Group. Annals of Internal Medicine. 1995;122:569–572. doi: 10.7326/0003-4819-122-8-199504150-00002. [DOI] [PubMed] [Google Scholar]
- Specks U, Fervenza FC, McDonald TJ, Hogan MCE. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis and Rheumatism. 2001;44:2836–2840. doi: 10.1002/1529-0131(200112)44:12<2836::aid-art471>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Stasi R, Pagano A, Stipa E, Amadori S. Rituximab chimeric anti-CD20 monoclonal antibody treatment for adults with chronic idiopathic thrombocytopenic purpura. Blood. 2001;98:952–957. doi: 10.1182/blood.v98.4.952. [DOI] [PubMed] [Google Scholar]
- Trape G, Fianchi L, Lai M, Laurenti L, Piscitelli R, Leone G, Pagano L. Rituximab chimeric anti-CD20 monoclonal antibody treatment for refractory hemolytic anemia in patients with lymphoproliferative disorders. Haematologica. 2003;88:223–225. [PubMed] [Google Scholar]
- Tsai HM. Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura. Journal of the American Society of Nephrology. 2003;14:1072–1081. doi: 10.1097/01.asn.0000060805.04118.4c. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. New England Journal of Medicine. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HM, Shulman K. Rituximab induces remission of cerebral ischemia caused by thrombotic thrombocytopenic purpura. European Journal of Haematology. 2003;70:183–185. doi: 10.1034/j.1600-0609.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Li A, Rock G. Inhibitors of von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura. Clinical Laboratory. 2001;47:387–392. [PubMed] [Google Scholar]
- Zaja F, Iacona I, Masolini P, Russo D, Sperotto A, Prosdocimo S, Patriarca F, de Vita S, Regazzi M, Baccarani M, Fanin R. B-cell depletion with rituximab as treatment for immune hemolytic anemia and chronic thrombocytopenia. Haematologica. 2002;87:189–195. [PubMed] [Google Scholar]
- Zaja F, Vianelli N, Sperotto A, De Vita S, Iacona I, Zaccaria A, Masolini P, Tomadini V, Tani M, Molinari AL, Baccarani M, Fanin R. B-cell compartment as the selective target for the treatment of immune thrombocytopenias. Haematologica. 2003a;88:538–546. [PubMed] [Google Scholar]
- Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G, Michelutti A, Baccarani M, Fanin R, Ferraccioli G. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003b;101:3827–3834. doi: 10.1182/blood-2002-09-2856. [DOI] [PubMed] [Google Scholar]
- Zecca M, Nobili B, Ramenghi U, Perrotta S, Amendola G, Rosito P, Jankovic M, Pierani P, De Stefano P, Bonora MR, Locatelli F. Rituximab for the treatment of refractory autoimmune hemolytic anemia in children. Blood. 2003;101:3857–3861. doi: 10.1182/blood-2002-11-3547. [DOI] [PubMed] [Google Scholar]
- Zheng X, Pallera AM, Goodnough LT, Sadler JE, Blinder MA. Remission of chronic thrombotic thrombocytopenic purpura after treatment with cyclophosphamide and rituximab. Annals of Internal Medicine. 2003;138:105–108. doi: 10.7326/0003-4819-138-2-200301210-00011. [DOI] [PubMed] [Google Scholar]