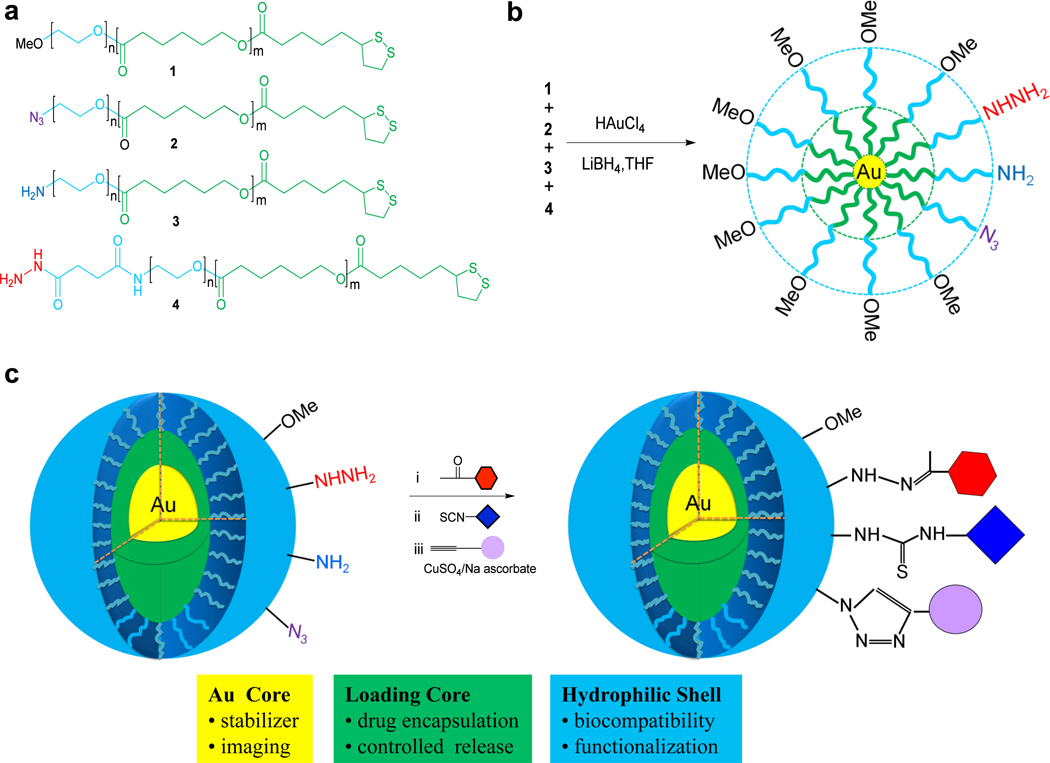
Figure 1.
Chemical synthesis of multifunctional gold- stabilized nanoparticles. The structure of block polymers modified at the apolar PCL end with thioic acid moiety for attachment to a gold core and at the PEO terminus with a functional group for post synthesis modification (a). The chemical synthesis of multifunctional nanoparticles by a modified Brust method (b). A cartoon of the architecture of the nanopaticles in aqueous solution and the three sequential bioorthogonal reactions for surface modification (c). The nanoparticles have an inner gold core (yellow) that provides stabilization, controlled degradation and and opportunity for imaging with TEM. The apolar PCL layer (green) provides a loading space for apolar compounds and the hydrophilic PEO shell (blue) offers biocompatibility. Three different surface functional groups are present for post synthesis modification. First, the acylhydrazides react selectively with ketones to give a hydrazone-linked modules. Next, the amines react with isocyanates or actived ester at pH 8–9 to give thioureas or amides, respectively and finally, azides are reacted with alkynes in the presence of Cu(I) to provide triazole-linked modules.