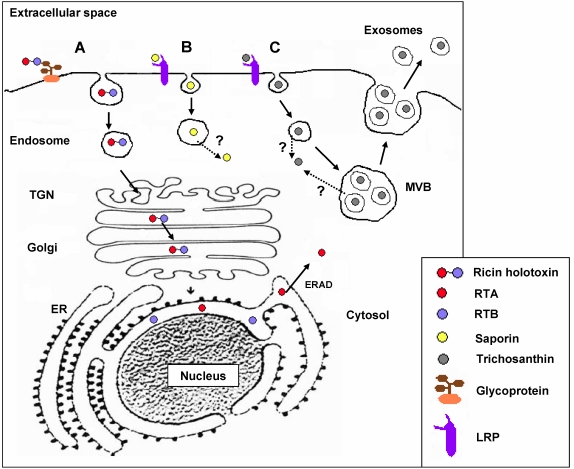
Figure 4.
Schematic representation of the intoxication pathways followed by Ricin (A), saporin (B) and trichosanthin (C). (A) Ricin binds to cell surface glycoproteins and after endocytosis is transported through endosomes to lysosomes (not indicated) or following endosomal sorting steps to the Trans Golgi Network (TGN) [81] and then retrogradely transported from the Golgi complex (passing through sulfation compartments) to the ER [82], where the A chain retrotranslocates to the cytosol via ERAD pathways: RTA has been shown to interact with negatively charged lipid vesicles and with ER membranes, undergoing a conformational change making it a better substrate for the ERAD system [33,83]. The molecular details of cytosolic entry via an ERAD-specific membrane E3 ubiquitin ligase have also been revealed showing how RTA may avoid ubiquitylation and proteasome degradation [84]. Finally, after escaping degradation RTA may refold in the cytosolic compartment also thanks to the presence of host cell chaperones [85]. (B) Saporin binding to cell surface is at least in part mediated by low-density lipoprotein receptor-related protein (LRP) and after being endocytosed, the toxin reaches the endo-lysosomal compartment from where it is delivered to the cytosol following as yet unidentified pathway(s). (C) TC binding to cell membranes can also be mediated by LRP members and can be delivered to multivesicular body (MVB) and in part incorporated into the intraluminal vesicles of this organelle. Fusion of the MVB to the plasma membrane allows release of the intraluminal vesicles into the extracellular space where they diffuse and can target other syngeneic or allogeneic cells through unknown mechanism(s).