Abstract
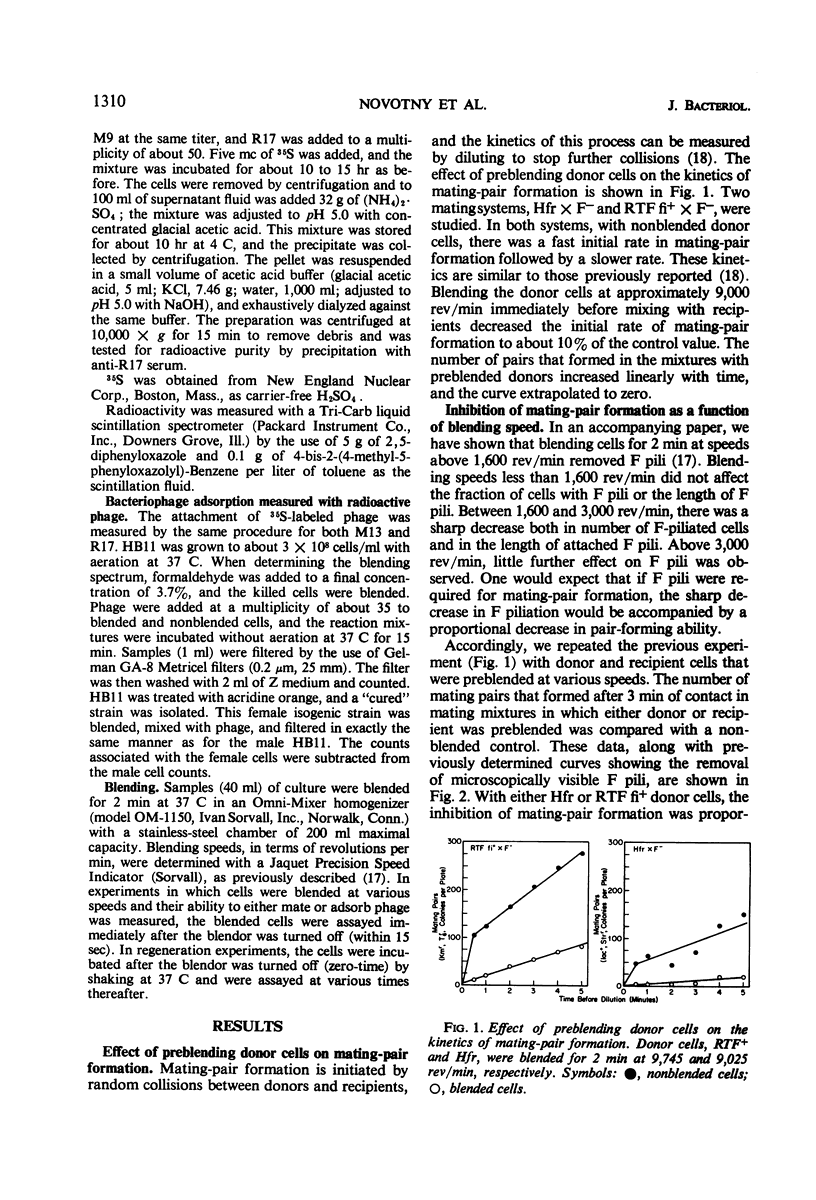
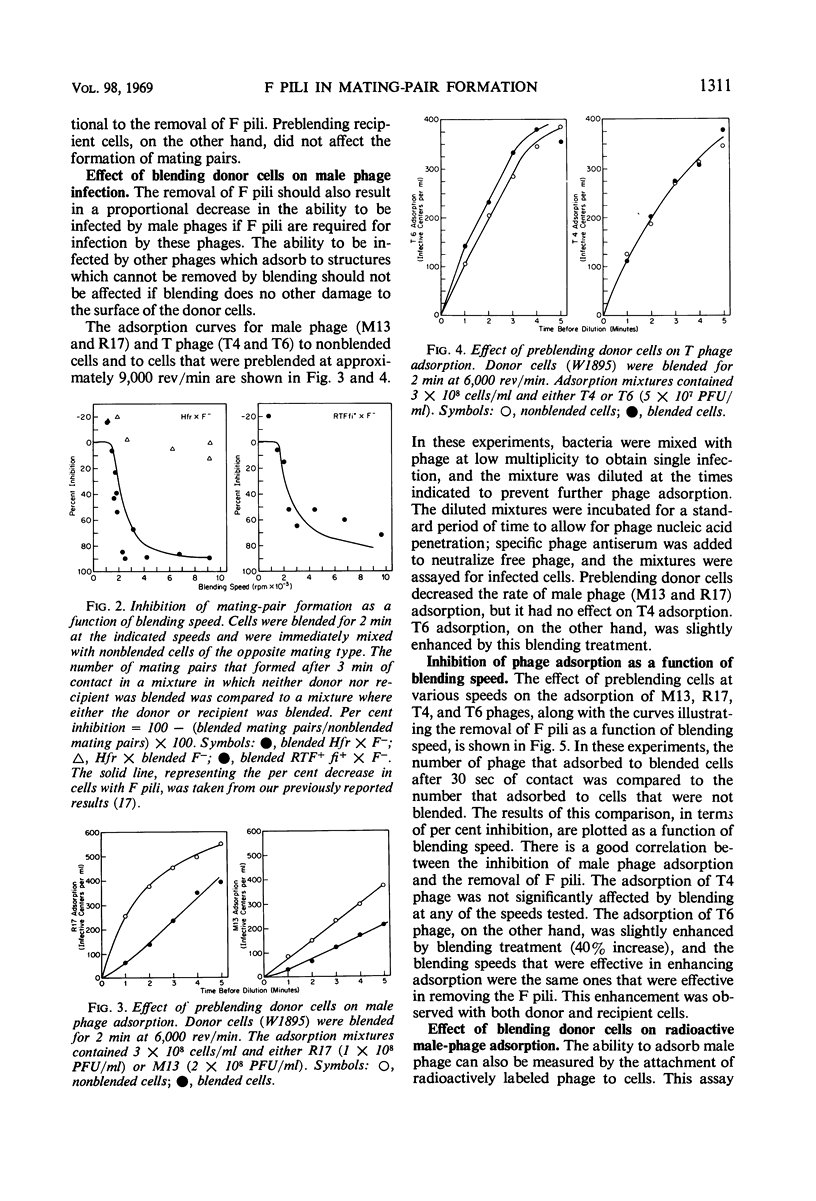
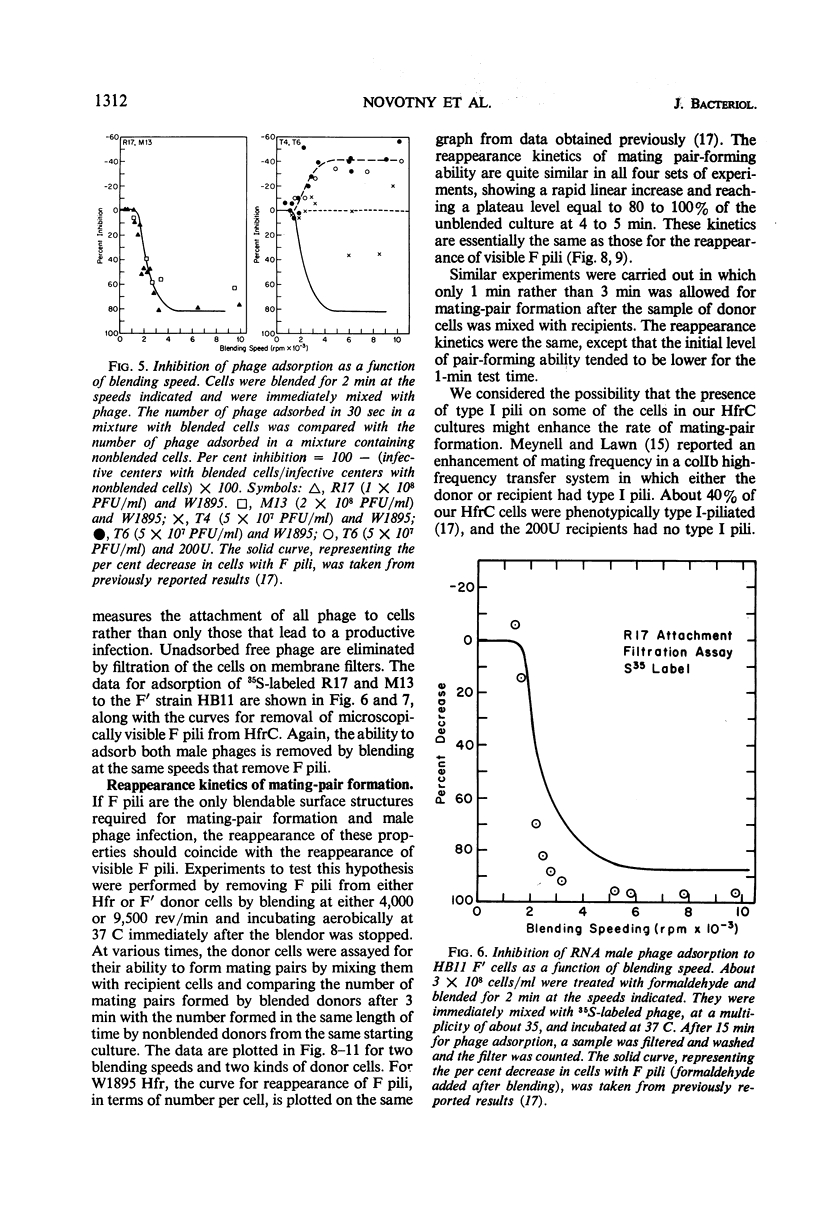
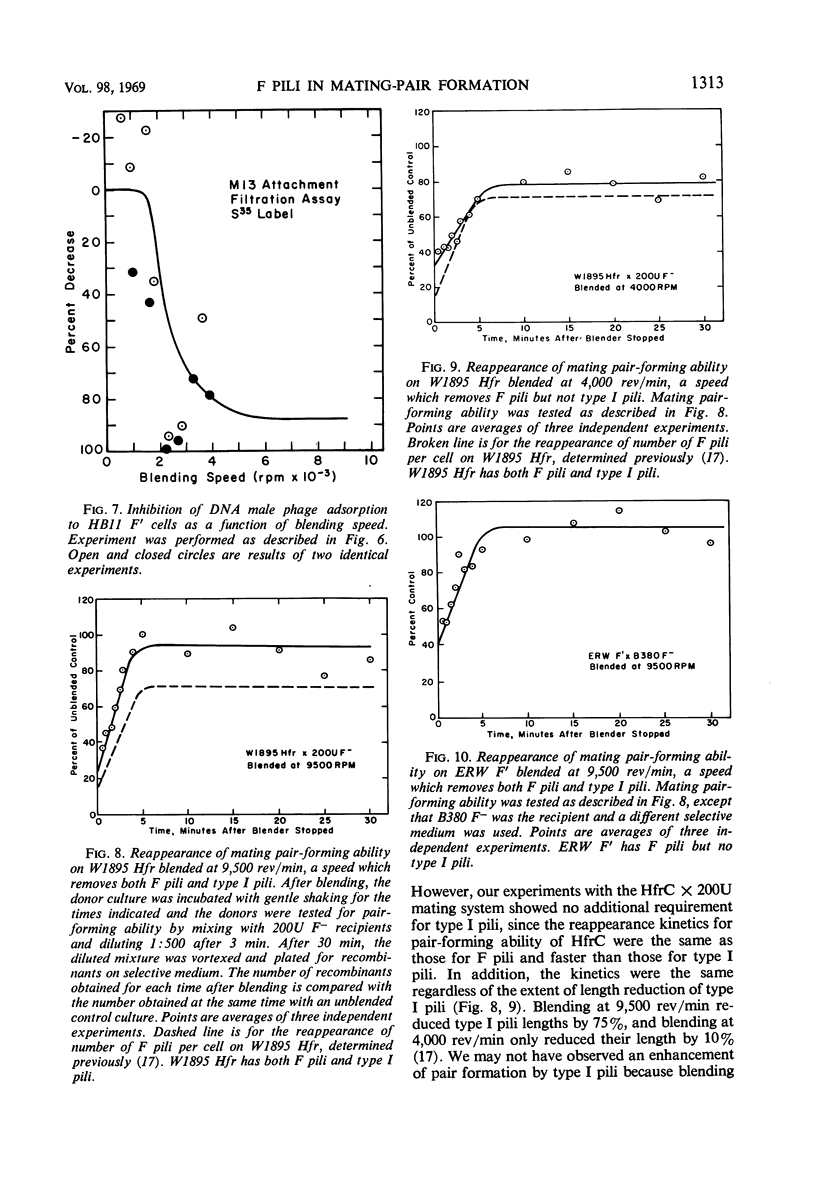
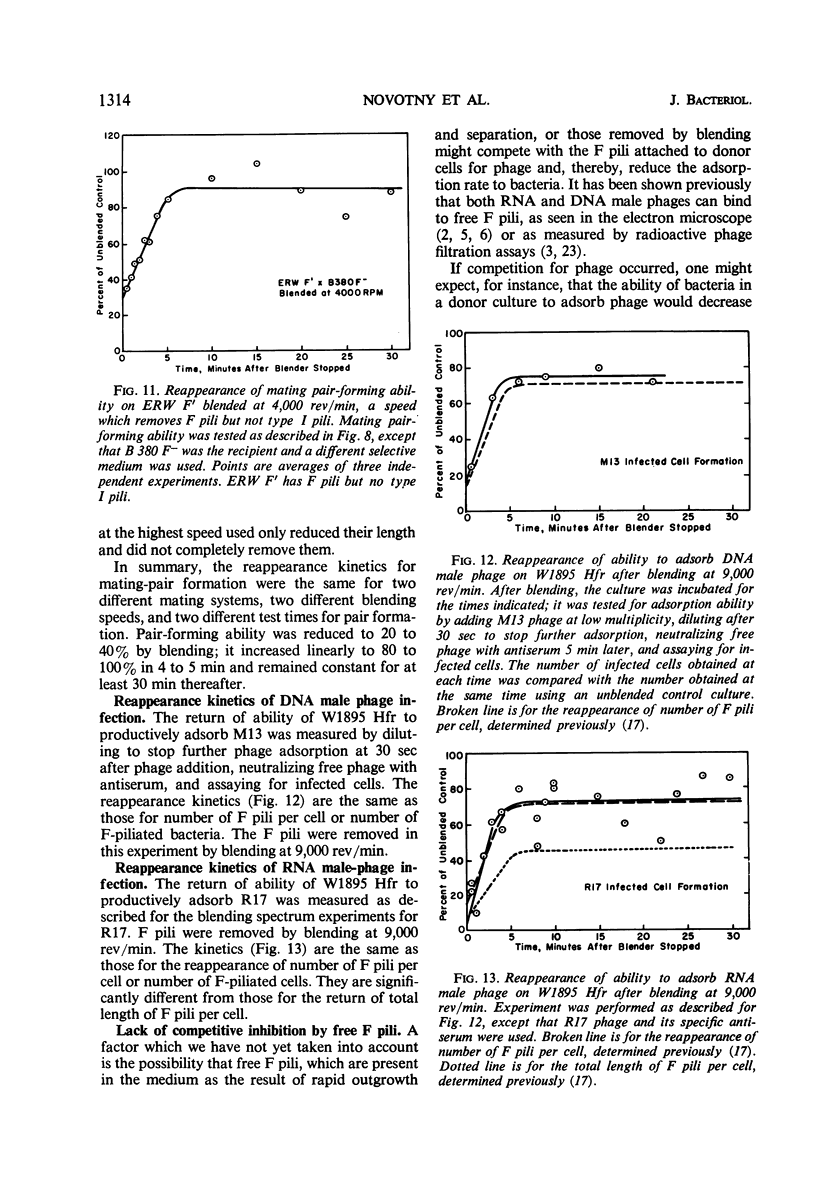
The extent of removal at various blending speeds (blending spectrum) and the kinetics of reappearance after blending of the ability of male Escherichia coli bacteria to form mating pairs, to adsorb and be infected by ribonucleic acid male phage, and to adsorb and be infected by deoxyribonucleic male phage were identical to the blending spectrum and reappearance kinetics of microscopically visible F pili. The same results were obtained with an Hfr (high-frequency recombinant), F′, or resistance transfer factor (RTF) fi+ mating system. Blending did not affect the viability, growth rate, ability to adsorb T4 phage, or ability to produce new F pili at any of the speeds used. It can be concluded that microscopically visible F pili are an absolute requirement for all three functions. Three classes of F pili have been found in bacterial cultures: attached, adsorbed, and free. Bacteria with adsorbed F pili in addition to attached ones were proportionately more susceptible to male phage infection, suggesting that adsorbed F pili may be at least partially functional. Free F pili did not compete with bacteria for phage. Some implications of the virus-like nature of F-pilus outgrowth for the mechanisms of mating and male phage infection are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINTON C. C., Jr, BUZZELL A., LAUFFER M. A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954 Dec;15(4):533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Sex pili and common pili in the conjugational transfer of colicin factor Ib by Salmonella typhimurium. Genet Res. 1967 Jun;9(3):359–367. doi: 10.1017/s0016672300010636. [DOI] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- Scaife J. Episomes. Annu Rev Microbiol. 1967;21:601–638. doi: 10.1146/annurev.mi.21.100167.003125. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Mobach H. W., Valentine R. C. Sex hair (F-pili) mutants of E. coli. Biochem Biophys Res Commun. 1967 May 5;27(3):412–416. doi: 10.1016/s0006-291x(67)80115-3. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., STRAND M. COMPLEXES OF F-PILI AND RNA BACTERIOPHAGE. Science. 1965 Apr 23;148(3669):511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]



