Abstract
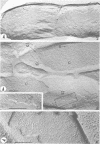
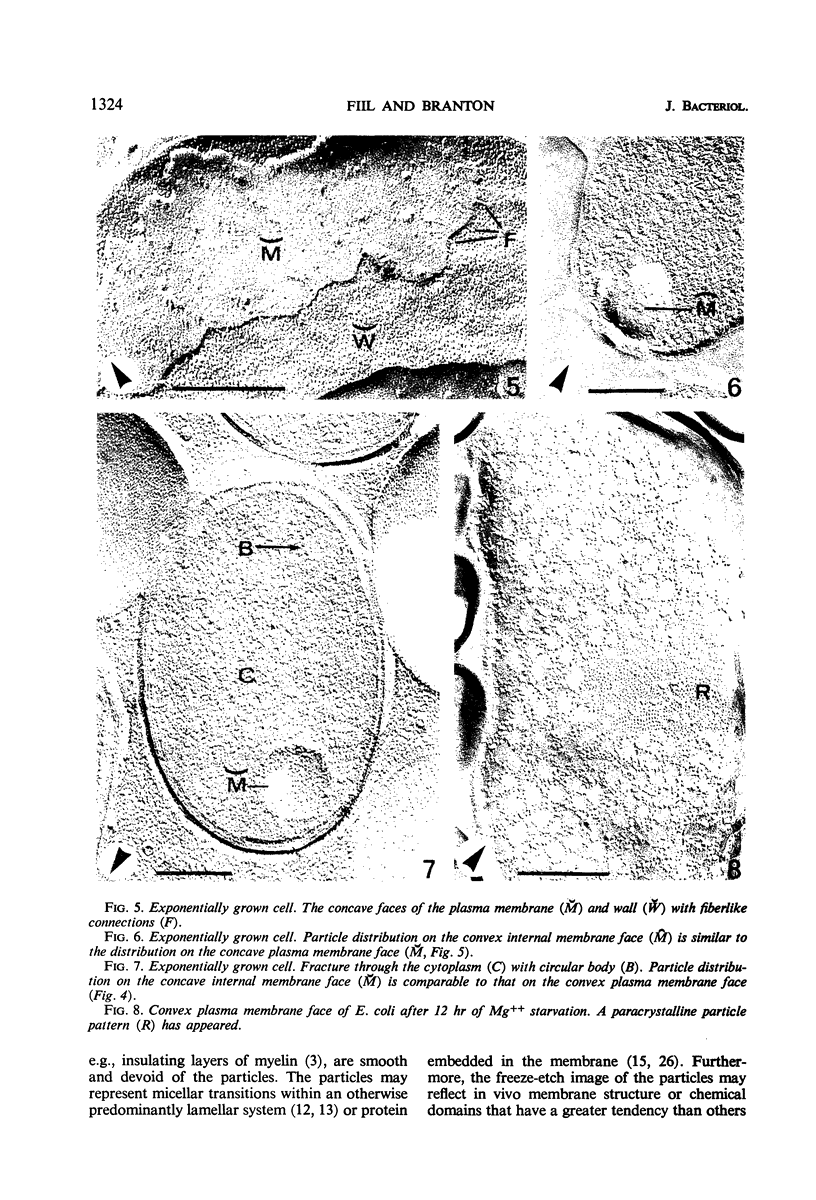
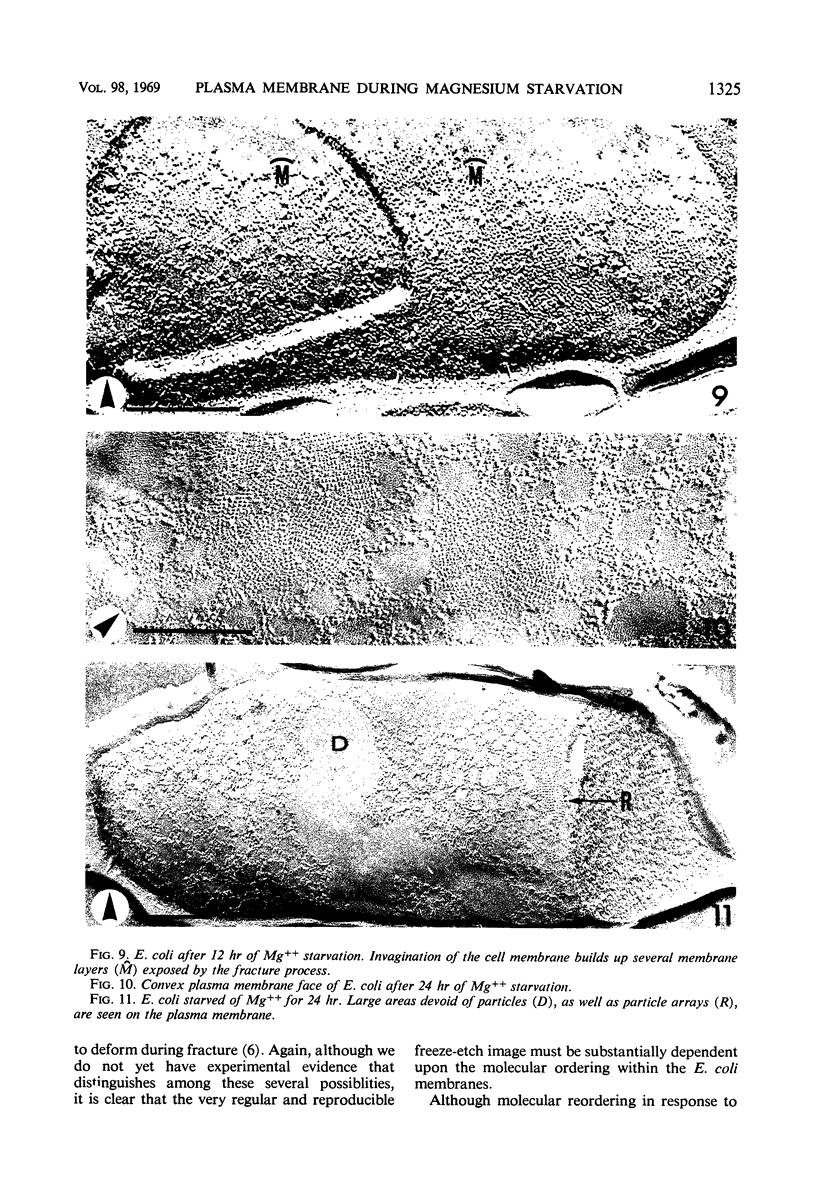
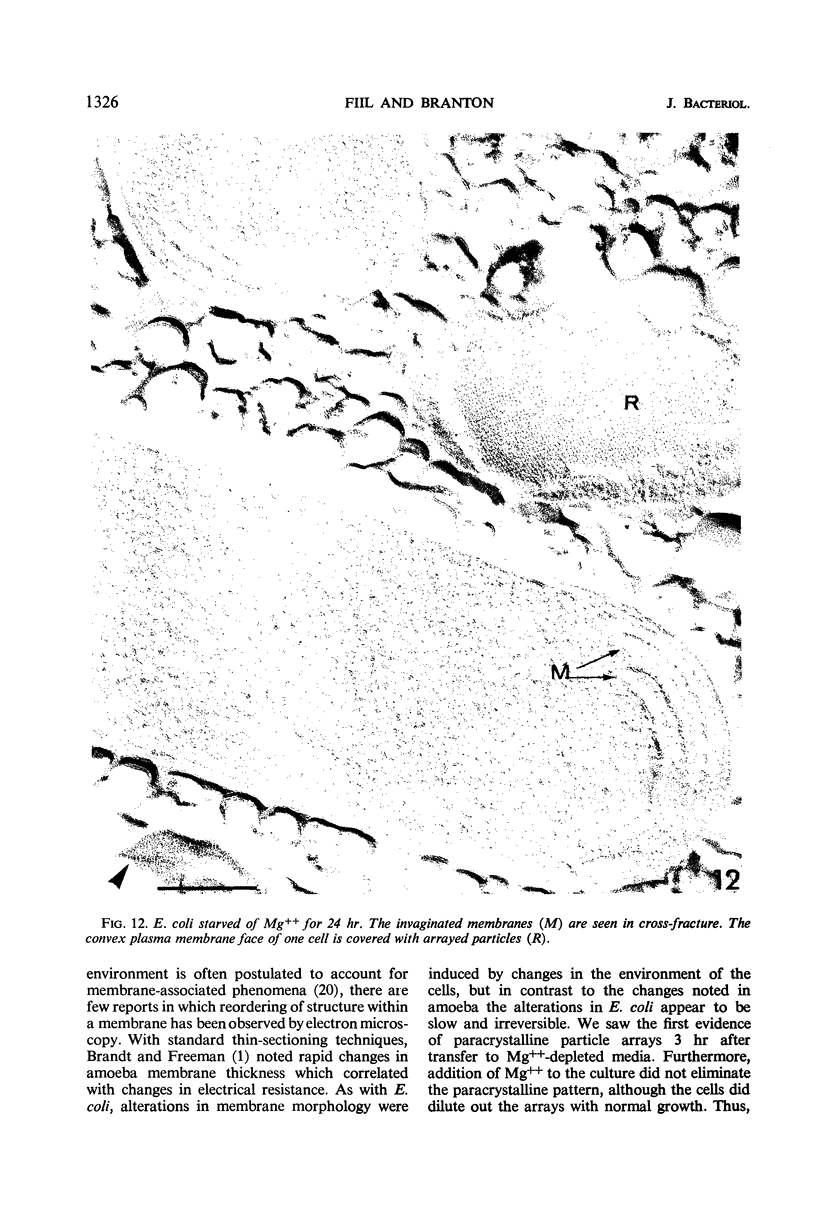
The effect of Mg++ starvation on the structure of the Escherichia coli cell membrane was studied with the freeze-etch technique. Special attention was paid to changes within the plane of the membrane, which in normal exponentially growing cells has a netlike arrangement of particles 2 to 6 nm in diameter. During Mg++ starvation, a paracrystalline particle pattern appeared on the plasma membrane, and large areas devoid of particles were seen. Although these changes are reproducibly associated with Mg++ starvation of the bacteria, no decrease in the Mg++ content of the cell envelope per se was detected, even after 24 hr of Mg++ deprivation. The structural changes caused by Mg++ deprivation appeared to involve specific and permanent alterations in membrane development. The absence of other nutrients or divalent cations did not induce similar alterations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D. Effects of magnesium ion deficiency on Escherichia coli and possible relation to the mode of action of novobiocin. J Bacteriol. 1962 Oct;84:679–682. doi: 10.1128/jb.84.4.679-682.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Freeman A. R. Plasma membrane: substructural changes correlated with electrical resistance and pinocytosis. Science. 1967 Feb 3;155(3762):582–585. doi: 10.1126/science.155.3762.582. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen myelin. Exp Cell Res. 1967 Mar;45(3):703–707. doi: 10.1016/0014-4827(67)90175-9. [DOI] [PubMed] [Google Scholar]
- Branton D., Park R. B. Subunits in chloroplast lamellae. J Ultrastruct Res. 1967 Aug;19(3):283–303. doi: 10.1016/s0022-5320(67)80222-3. [DOI] [PubMed] [Google Scholar]
- Clark A. W., Branton D. Fracture faces in frozen outer segments from the guinea pig retina. Z Zellforsch Mikrosk Anat. 1968;91(4):586–603. doi: 10.1007/BF00455276. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Ennis H. L. Amino acid regulation of RNA synthesis during recovery of Escherichia coli from Mg2+ starvation. Biochim Biophys Acta. 1967 Sep 26;145(2):300–309. doi: 10.1016/0005-2787(67)90048-2. [DOI] [PubMed] [Google Scholar]
- Cota-Robles E. H. Internal membranes in cells of Escherichia coli. J Ultrastruct Res. 1966 Dec;16(5):626–639. doi: 10.1016/s0022-5320(66)80010-2. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Branton D. Fracture planes in an ice-bilayer model membrane system. Science. 1967 Nov 3;158(3801):655–657. doi: 10.1126/science.158.3801.655. [DOI] [PubMed] [Google Scholar]
- Kavanau J. L. Membrane structure and function. Fed Proc. 1966 May-Jun;25(3):1096–1107. [PubMed] [Google Scholar]
- LUCY J. A. GLOBULAR LIPID MICELLES AND CELL MEMBRANES. J Theor Biol. 1964 Sep;7:360–373. doi: 10.1016/0022-5193(64)90080-3. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. A general theory of membrane transport from studies of bacteria. Nature. 1957 Jul 20;180(4577):134–136. doi: 10.1038/180134a0. [DOI] [PubMed] [Google Scholar]
- MOOR H., MUHLETHALER K., WALDNER H., FREY-WYSSLING A. A new freezing-ultramicrotome. J Biophys Biochem Cytol. 1961 May;10:1–13. doi: 10.1083/jcb.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Chan B., Rose H. M. Electron microscopy of magnesium-depleted bacteria. J Bacteriol. 1966 Feb;91(2):891–895. doi: 10.1128/jb.91.2.891-895.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penniston J. T., Green D. E. The conformational basis of energy transformations in membrane systems. IV. Energized states and pinocytosis in erythrocyte ghosts. Arch Biochem Biophys. 1968 Nov;128(2):339–350. doi: 10.1016/0003-9861(68)90040-4. [DOI] [PubMed] [Google Scholar]
- Remsen C. C. Fine structure of the mesosome and nucleoid in frozen-etched Bacillus subtilis. Arch Mikrobiol. 1968;61(1):40–47. doi: 10.1007/BF00704290. [DOI] [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEERE R. L. Electron microscopy of structural detail in frozen biological specimens. J Biophys Biochem Cytol. 1957 Jan 25;3(1):45–60. doi: 10.1083/jcb.3.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R. S., Bullivant S. The application of freeze-cleaving technics to studies on red blood cell fine structure. Blood. 1967 May;29(5):780–789. [PubMed] [Google Scholar]