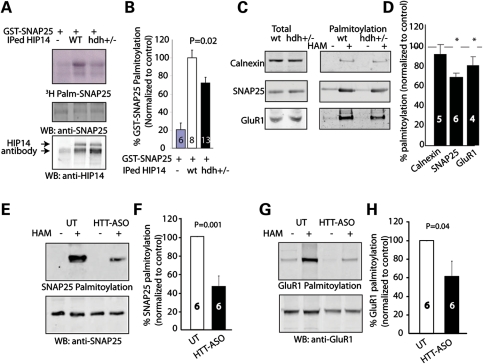
Figure 6.
Loss of wild-type HTT leads to palmitoylation defects of SNAP25 and GluR1 in vivo. (A) Representative image shows IPed HIP14 of hdh+/− mice brain exhibited less enzymatic activity to palmitoylate GST-SNAP25, compared with that of wild-type mice. HIP14 antibody bound to sepharose beads was used to IP HIP14 from whole brain lysate of wild-type or hdh+/− mice. Then the beads containing IgG control or IPed HIP14 were added to reaction mix containing 3H-palmitoyl-CoA to palmitoylate GST-SNAP25 in vitro. (B) Quantification revealed that IPed HIP14 of hdh+/− mice brain had ∼30% reduction in its enzymatic activity. (C and D) ABE palmitoylation assay was performed on wild-type and hdh+/− mice brain to determine palmitoylation level of neuronal proteins. After ABE palmitoyl-protein purifications of wild-type and hdh+/− mice, extracted palmitoylated proteins were subject to protein electrophoresis and western blotting with calnexin, SNAP25 or GluR1 antibodies. Quantification and representative blots showed alteration in palmitoylation of SNAP25 (80.6 ± 8.4%) and GluR1 (79.4 ± 3.8%) in hdh+/− brain compared with the wild-type (100%). (E–H) In HTT-ASO-treated mouse cortical neurons, palmitoylation of SNAP25 (46.4 ± 11.8%) and GluR1 (61.6 ± 16.7%) was further reduced as determined by ABE palmitoylation assay described in (C) and (D). Number of repeats was indicated in the graph.