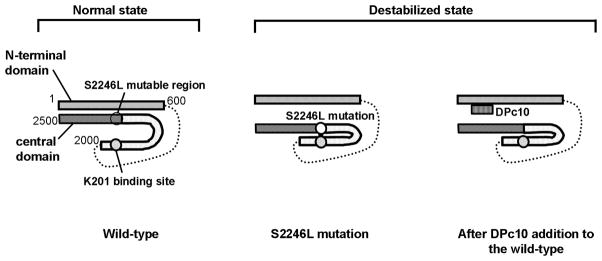
Figure 8.
Hypothetical model showing how CPVT-associated mutation causes a drastic change in the channel function by mediation of defective inter-domain interactions within RyR2.
In the ‘normal state’ of RyR2, tight interaction between the N-terminal domain and the central domain (zipped domain switch) is coupled with a loose interaction (unzipping) between the two sub-domains of the central domain [the 2246 domain (S2246L mutable region) and the K201-binding domain], then the closed state of the channel is maintained. In the ‘destabilized state’, the S2246L mutation in the 2246 domain induces an abnormally tight interaction of the S2246 domain/K201-binding domain pair. This produces domain unzipping of the N-terminal domain/central domain pair (the domain switch), and erroneous activation of the channel results in diastolic Ca2+ leak. Addition of DPc10, which interferes with a tight interaction between the N-terminal and central domains, to the wild type RyR2 produces domain unzipping of the domain switch, mimicking the situation caused by CPVT mutation (R2474S, S2246L).