Figure 2.
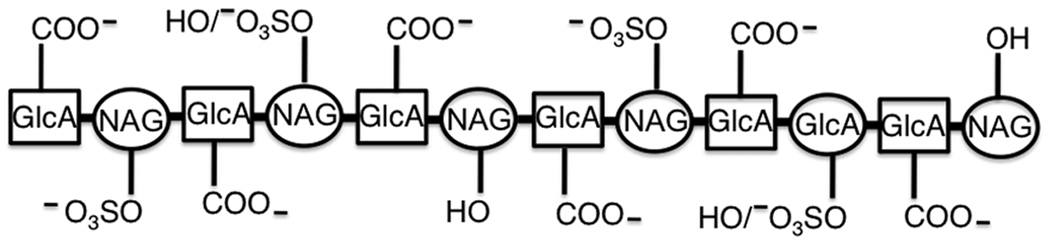
Schematic representation of a C4S dodecasaccharide. Based on the specificity of IRBC binding to C4S, it is inferred that the carboxyl groups of glucuronic acid (GlcA) interact with VAR2CSA. Although the carboxyl group of the GlcA residue at the non-reducing terminal end appears to be required for binding whether all of the reminder carboxyl groups or only some of them interact is not known. Given that dodecasaccharides containing 2, 3, 4 sulfate exhibit similar levels of efficient inhibitory activity whereas those containing 1 or 5 sulfate groups have substantially lower inhibitory activity, it is logical that two hydroxyl groups at the C-4 position of N-acetylgalatosamine (NAG) interact with VAR2CSA. The N-acetyl groups do not interact, but it is possible that the nitrogen lone pair of electrons is also involved. C4S assumes single-stranded helical conformation and therefore, the schematic structure shown here does not accurately represent the spatial orientations of the depicted functional groups.
