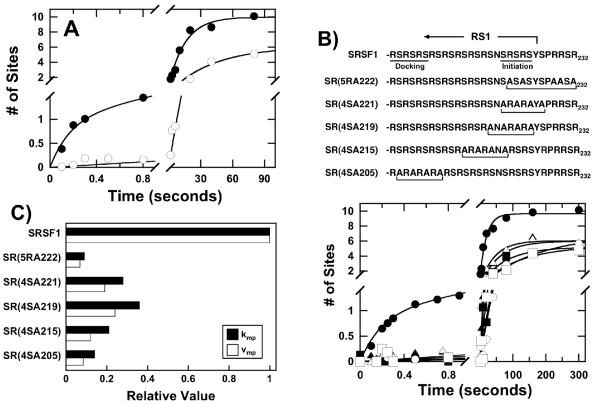
Figure 3. Enzyme-Substrate Interactions Support Phosphorylation Rates.
A) Docking Groove. The phosphorylation of SRSF1 by a form of SRPK1 containing 6 mutations in the docking groove [SRPK1(6M)] was studied. The data for wild-type SRPK1 (●) are fit to values of 5.7 sec−1 for kp, 0.063 for kmp, 1 site for αp, and 9 sites for αmp. For SRPK1(6M) (○) the data are fit to single exponential function with values of 0.023 sec−1 for kmp and 6.1 sites for αmp. B) RS Domain. The phosphorylation of multiple Arg-to-Ala and Ser-to-Ala mutants in the RS domain of SRSF1 by SRPK1 were studied. For wild-type SRSF1 (●), values of 5 sec−1 for kp, 0.050 sec−1 for kmp, 1 site for αp and 8.7 sites for αmp are obtained. The data for the mutants are fit to single exponential functions with values of 0.0057 sec−1 (kmp) and 6.9 sites (αmp) for SR(5RA222) (○), 0.018 sec−1 (kmp) and 6.0 sites (αmp) for SR(4SA221) (▲), 0.023 sec−1 (kmp) and 6.0 sites (αmp) for SR(4SA219) (△), 0.013 sec−1 (kmp) and 5.2 sites (αmp) for SR(4SA215) (■), 0.009 sec−1 (kmp) and 5.3 sites (αmp) for SR(4SA205) (□). C) Multi-Site Phosphorylation. The bar graph displays the ratio of kmp and vmp (kmp x αmp) for the mutants compared to SRSF1.