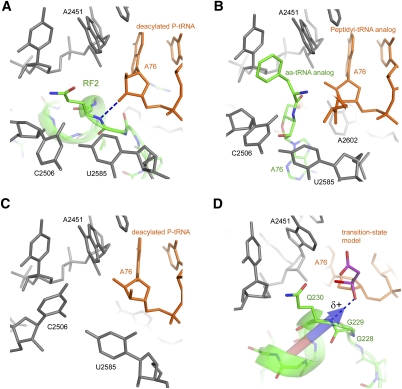
FIGURE 5.
Conformation of the peptidyl-transferase center depends on the occupancy of the A site. The conformations of the PTC are similar in ribosome complexes, in which the A site is occupied by (A) a release factor (Korostelev et al. 2008) and (B) aminoacyl-tRNA (Voorhees et al. 2009). (C) C2506 and U2585 are found in a different conformation when the 50S subunit A-site is vacant (Selmer et al. 2006). (D) Superposition of a peptidyl-transferase transition-state analog complexed with the 50S subunit (Schmeing et al. 2005a) on the structure of the 70S termination complex (Laurberg et al. 2008). The main-chain amide of Gln230 is positioned to H-bond with the oxyanion of the transition state. The dipole moment of the a-helix likely increases the partial positive charge on the Gln230 backbone NH group and the ability of the NH group to stabilize the negatively charged transition-state intermediate. The dipole moment vector was calculated at Protein Dipole Moments Server (Felder et al. 2007).