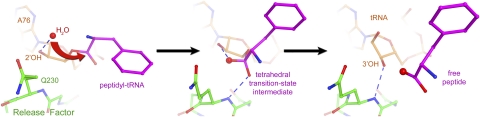
FIGURE 6.
Scheme for the mechanism of the peptidyl-tRNA hydrolysis reaction. (Left panel) Nucleophilic attack. The nucleophilic water molecule is positioned for nucleophilic attack at H-bonding distance of the 2′-OH of ribose 76 of the peptidyl-tRNA (Jin et al. 2010). (Center panel) Transition-state stabilization. The oxyanion of the developing tetrahedral transition state is stabilized by the hydrogen bond with the backbone amide NH group of Gln230. (Right panel) Product stabilization. Following hydrolysis, the 3′-hydroxyl leaving group of the deacylated P-site tRNA H-bonds with the backbone amide NH group of Gln230. Crystal structures of RF2-bound 70S complexes in the presence of a peptidyl-tRNA analog (Jin et al. 2010) and deacylated tRNA (Korostelev et al. 2008) were used in the left and right panels, respectively. The transition state (center panel) was modeled by superimposing the 23S rRNA structure of the RF2 termination complex (Korostelev et al. 2008) with the structure of a 50S subunit containing a transition-state analog (Schmeing et al. 2005a). (Adapted, with modifications, from Korostelev et al. 2010.)